Oxygen-induced lung injury is believed to lead to the development of bronchopulmonary dysplasia in premature infants. We have evaluated the beneficial effects of Nigella sativa oil (NSO) on rats with hyperoxia-induced lung injury.
MethodsThirty newborn Sprague-Dawley rats were randomly divided into 3 groups as hyperoxia (95% O2), hyperoxia+NSO and control (21% O2). Pups in the hyperoxia+NSO group were administered intraperitoneal NSO at a dose of 4ml/kg daily during the study period. Histopathologic, immunochemical, and biochemical evaluations (superoxide dismutase [SOD], glutathione peroxidase [GSH-Px], malonaldehyde [MDA] and myeloperoxidase [MPO]) were performed.
ResultsIn the histopathologic and immunochemical evaluation, severity of lung damage was significantly lower in the hyperoxia+NOS group (P<.05). Tissue GSH-Px and SOD levels were significantly preserved, and MDA, MPO levels were significantly lower in the hyperoxia+NSO group (P<.05).
ConclusionNSO significantly reduced the severity of lung damage due to hyperoxia.
Se cree que la lesión pulmonar inducida por el oxígeno conduce al desarrollo de una displasia broncopulmonar en los recién nacidos prematuros. Hemos evaluado los efectos favorables del aceite de Nigella sativa (NSO) en ratas con lesión pulmonar inducida por hiperoxia.
MétodosSe utilizaron 30 ratas Sprague-Dawley recién nacidas a las que se dividió aleatoriamente en 3 grupos para aplicarles hiperoxia (O2 al 95%), hiperoxia+NSO o el grupo de control (O2 al 21%). A las crías del grupo de hiperoxia+NSO se les administró NSO a una dosis de 4ml/kg al día por vía intraperitoneal durante el periodo de estudio. Se realizó una evaluación histopatológica, inmunoquímica y bioquímica (superóxido dismutasa [SOD], glutatión peroxidasa [GSH-Px], malonilaldehído [MDA] y mieloperoxidasa [MPO]).
ResultadosEn la evaluación histopatológica e inmunoquímica, la gravedad de la lesión pulmonar fue significativamente inferior en el grupo de hiperoxia+NOS (p<0,05). Los niveles tisulares de GSH-Px y SOD se mantuvieron significativamente preservados, y los niveles de MDA y MPO fueron significativamente inferiores en el grupo de hiperoxia+NSO (p<0,05).
ConclusiónEl NSO redujo significativamente la gravedad de la lesión pulmonar debida a la hiperoxia.
Bronchopulmonary dysplasia (BPD) is a chronic pulmonary disease seen in premature newborns who required mechanical ventilation and oxygen therapy due to acute respiratory distress.1,2 Although its etiopathogenesis is not completely understood, it is believed to be multifactorial, influenced by prematurity, oxidative lung lesion and inflammation, like chorioamnionitis, hyperoxia, infection, respirator-induced injury and many other adverse stimuli. The lesion alters the normal growth and maturation of the lungs caused by deteriorated alveolar and vascular growth, leading to the abnormal lung structure of BPD.2–4 It was reported for the first time more than 40 years ago, but the nature of BPD has been modified with the increasing use of prenatal corticosteroids, surfactant therapy, new ventilatory strategies and other treatments.5,6 Nonetheless, BPD continues to be an important cause of neonatal morbidity and mortality, as improved perinatal care has increased the survival rate of extremely premature newborns (<28 weeks of gestation).2,4,7 These children are at high risk for having long-term lung injury due to the significant deterioration in lung function produced by the prolonged use of respirators during neonatal intensive care unit stays. After hospital discharge, these children may also have frequent hospitalizations, recurring respiratory exacerbations, intolerance to exercise and other signs of late cardiorespiratory disease, in addition to adverse neurodevelopmental evolution.4 Clinical and laboratory studies suggest that early intervention strategies that directly preserve survival and lung cell function should prevent the appearance of BPD.3,7 However, we still do not have treatments that can effectively attenuate lung lesions and promote lung growth in order to reduce the incidence and severity of BPD. As a consequence, it is necessary to identify new treatment options for the prevention of severe pulmonary injury in the course of BPD.
The Nigella sativa (NS) plant, commonly known as “black seed”, is used as a medicinal plant for the treatment of many diseases.8 In clinical and experimental studies, several therapeutic effects of NS and its extracts have been reported in respiratory diseases.9–12 In NS extracts, many enriched bioactive molecules have been identified, both in fixed oil as well as essential oil. It has been demonstrated that much of the biological activity of NS is due to thymoquinone, which is the main component of the essential oil and fixed oil.8 It has been observed that the volatile oil contains 18.4%–24% thymoquinone and also 46% monoterpenes (e.g. p-cymene, a-pinene).13 Several papers have identified that the extracts of NS have anti-inflammatory and antimicrobial properties (against several germs) as well as antioxidant properties due to its activity of free-radical elimination.14 In addition, its inhibitory action of human neutrophil elastase has been determined15 along with its immunomodulation and cytoprotection activity in many systems of the body with a low grade of toxicity in clinical and experimental studies.8,16 However, the effects of NS oil (NSO) on lungs that have undergone hyperoxic injury have not yet been researched. The objective of this study was therefore to investigate the favorable effects of NSO on the lungs of rat pups with BPD induced by hyperoxia.
Material and MethodsExperimental DesignThe study was approved by the Ethics Committee for animal experimentation studies at the GATA Military School of Medicine (Ankara, Turkey). National Research Council guidelines were followed for the care and use of laboratory animals. Four pregnant Sprague-Dawley rats were used, which spontaneously gave birth. Afterwards, all the pups were grouped, randomly distributed and then returned to the mother rats. Thirty newborn rats were used and distributed into the 3 following groups: control, hyperoxia and hyperoxia with NSO treatment. The pups in the hyperoxia group were exposed to 95% O2, while the pups of the control group breathed room air with 21% O2. The mothers were rotated between the litters exposed to hyperoxia and room air every 24h in order to avoid oxygen toxicity in them. Continuous exposure to 95% O2 was reached in a Plexiglas chamber (70cm×60cm×30cm) with the use of a continuous flow system. The level of oxygen in the interior of the Plexiglas chamber was continuously monitored with a Ceramatec oxygen analyzer (MAXO2) in order to maintain O2 saturation ≥95%. The experimental pups of the control group (room air with 21% O2 for 10 days+placebo) and those with hyperoxia alone (95% O2 for 10 days+placebo) were administered intraperitoneal saline solution (4ml/kg), while the pups in the hyperoxia+NSO group (95% O2 for 10 days+NSO) were administered NSO (Origo [100% natural N. sativa seed oil], Gaziantep, Turkey) at a dose of 4ml/kg17 once a day with a microsyringe, intraperitoneally, from the first until the last day of the study. Before being administered, the NSO was sterilized by filtration with Bexen 0.2μm filters (Oiarso S. Coop., Guipuzkoa, Spain). It has been demonstrated that the different compositions present in NSO act synergically, and this suggests the importance of using the complete oil of the seeds in studies. Thus, in this study we have used whole oil.8 The pups were weighed daily on a scale with a sensitivity of 0.01g, and the weights were recorded.
Histopathologic, Morphologic and Immunochemical Analyses of the LungsAll the pups were sacrificed on the 10th day of the study under deep anesthesia with intraperitoneal ketamine/xylazine (100–10mg/kg). The thorax was opened, the lungs extracted and set with 4% paraformaldehyde perfusion (PFA) buffered with 0.1M phosphate (PBS). During perfusion, constant inflation pressure was maintained at 5cmH2O by means of a tracheal catheter. When the perfusion was completed, the trachea was ligated with a surgical suture and the lungs incubated in a 4% solution of cold PFA–PBS, with ice for 4–5h. After this incubation, the PFA–PBS solution was replaced with two quick changes of cold PBS to eliminate outer residue. Then, the lungs were transferred to a solution of 30% PBS/sucrose that was sterile and filtered, and they were maintained at 4°C until total equilibrium. The lungs were included in paraffin and 5μm slices were made from the paraffin blocks. Slices were selected using a systematic randomized sampling procedure, and they were mounted on slides coated with poly-l-lysine (Histobond adhesive slides, Marienfeld, Germany). The slides were stained with standard techniques for hematoxylin-eosin and Masson's trichrome for the histopathologic exam and with the ABC technique for the of lamellar body membrane protein.
For the immunohistochemical detection of lamellar body membrane protein, the slices were rehydrated and then treated with 13% hydrogen peroxide for 30min. Afterwards, the blocks underwent a non-specific block with goat serum for 30min and then were incubated with primary antibodies against membrane protein of lamellar bodies (1:100, Chemicon, AB3623-rabbit, USA) for a night at 4°C, followed by a treatment with biotinylated anti-rabbit secondary antibody (1:200, Vector Laboratories, Peterborough, United Kingdom) for 30min at room temperature. After the treatment with the avidin–biotin complex, 3,3′-diaminobenzidine was used (DAB; Vector Laboratories, Peterborough, United Kingdom) to reveal the color. Negative control preparations were also used, in which the primary anti-body was eliminated. The tissue slices were examined by optical microscopy with a digital camera. The positive cells for lamellar bodies (type II cells) of the alveolar area were counted 5 times in 10 slices with a separation amongst them of 25μm from each animal. The data were presented as number of cells positive for lamellar bodies (type II cells)/mm2.
The histopathologic exam and the immunohistochemistry score were carried out by a blinded, experienced pathologist who did not know the treatment group to which each sample belonged. The histopathologic score was established with the following grades: grade 1, normal histology; grade 2, moderate leukocyte infiltration; grade 3, leukocyte infiltration, edema and partial destruction; and grade 4, total tissue destruction.
The radial alveolar count was also evaluated in the slices by means of digital imaging in order to evaluate alveolar development. In brief, a line was drawn from the center of a respiratory bronchiole to the closest connective tissue partition at right angles with the epithelium, and the number of alveolar partitions that crossed the line was counted in 3–4 slices from each animal.18 The coefficients of the subepithelial and epithelial areas were obtained (which reflect cell infiltration and fibrosis) using morphometric evaluation of the lung tissue with the help of a computer program (Scion Image, 4.0.3) using a previously modified method.19 For this, cross-sectional airway slices (>100μm) were selected that had been stained with Masson's trichrome, and the interior perimeters of the epithelium, the perimeters of the basal membrane and the outer perimeters of the adventitia were plotted with the aid of a computer program (Scion Image, 4.0.3). The areas of these outlines slices were calculated in order to evaluate the subepithelial and epithelial area coefficients.
Biochemical AnalysisA biochemical analysis was done of the activity of malondialdehyde (MDA), myeloperoxidase (MPO), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in the left lung tissue of the study groups. The lung tissue obtained was perfused with physiological saline solution before homogenization in order to eliminate the residual blood. The tissues were homogenized in physiological saline solution (1g in 5ml) with the use of a homogenizer (IKA T18 basic Ultra-turrax, Germany) and they were centrifuged at 4000×g for 20min (NF 800 R Nüve). The clear supernatant was separated to be used in the analysis. The determinations were done by spectrophotometer (UV Shimadzu 1700, Japan). The protein determinations were done with the Lowry method.20
Determination of Superoxide Dismutase ActivityTotal SOD (Cu, Zn, Mn) activity was determined with the Sun et al. method.21 The principle of this method is based on inhibiting the reduction of nitro blue tetrazolium by the xanthine–xanthine oxidase system as a generator of superoxide. The activity of SOD is expressed as U/mg of protein.
Determination of Glutathione Peroxidase ActivityThe activity of glutathione peroxidase was determined with the Paglia and Valentine method.22 The enzyme reaction in the tube, with NADPH, reduced glutathione (GSH), sodium azide and glutathione reductase, was initiated with the addition of H2O2, and the change in absorbency to 340nm was monitored. The activity of GSH-Px is expressed in U/g of protein.
Determination of Malondialdehyde LevelsTissue MDA levels were determined with the Draper and Hadley method based on the reaction of MDA with thiobarbituric acid (TBA) at 95°C.23 In the reaction of TBA, MDA and TBA react to form a pink pigment with a maximum absorption of 532nm. The reaction was at a pH of 2–3 and 95°C for 15min. The sample was mixed with 2.5 volumes of 10% trichloroacetic acid (weight/volume) to precipitate the protein. The precipitate was separated by centrifuge and the supernatant was reacted with 0.67% TBA in a double-boiler for 15min. After cooling, the absorbency was read at 532nm (Shimadzu UV-1700, Japan). The arbitrary values obtained were compared with a series of standard reference solution (1,1,3,3-tetramethoxypropane). The results are expressed as nmol/mg of tissue.
Determination of Myeloperoxidase ActivityMyeloperoxidase activity was determined with the method based on the principle that a homogenized mixture that contains MPO activity reduces o-dianisidine dihydrochloride in the presence of H2O2, and this reduced product has an absorption of 460nm. MPO activity was calculated with the use of the extinction coefficient of o-dianisidine. The results were expressed in units per gram of tissue (U/g).24
Statistical AnalysisFor the statistical analysis, the SPSS (v15.0, IL, USA) program was used. Normal data distributions were examined graphically with the Shapiro–Wilks test. Comparisons of numerical variables were done with an ANOVA and the Bonferroni correction. Discrete variables were compared with a χ2 test. Differences with a P value <.05 were considered statistically significant. Data were expressed as mean±standard deviation (SD). The statistical power was analyzed. Taking a magnitude effect value of 0.60 and an alpha error of 0.05 and a beta error of 0.20 to obtain a statistical power of 80%, 10 animals were observed to be sufficient in each group.
ResultsAt the end of the experiment, 3 rat pups from the hyperoxia group and one from the hyperoxia+NSO group had died. However, there were no significant differences between the 2 groups regarding survival rate (P>.05). In addition, mean birth weight of the pups in the study groups was 4.9±0.4g in the control group, 5.0±0.3g in the hyperoxia group, and 5.1±0.2g in the hyperoxia+NSO group. No significant differences were observed in birth weight among the three groups (P>.05). At the end of the study, the mean bodyweight of the pups in the control group was 16.9±1.5g, but the mean weights in the hyperoxia+NSO and hyperoxia groups were 13.7±1.3g and 11.1±0.8g, respectively, and the mean weight of the pups from the hyperoxia+NSO group was significantly higher than that of the pups from the hyperoxia group (P<.05).
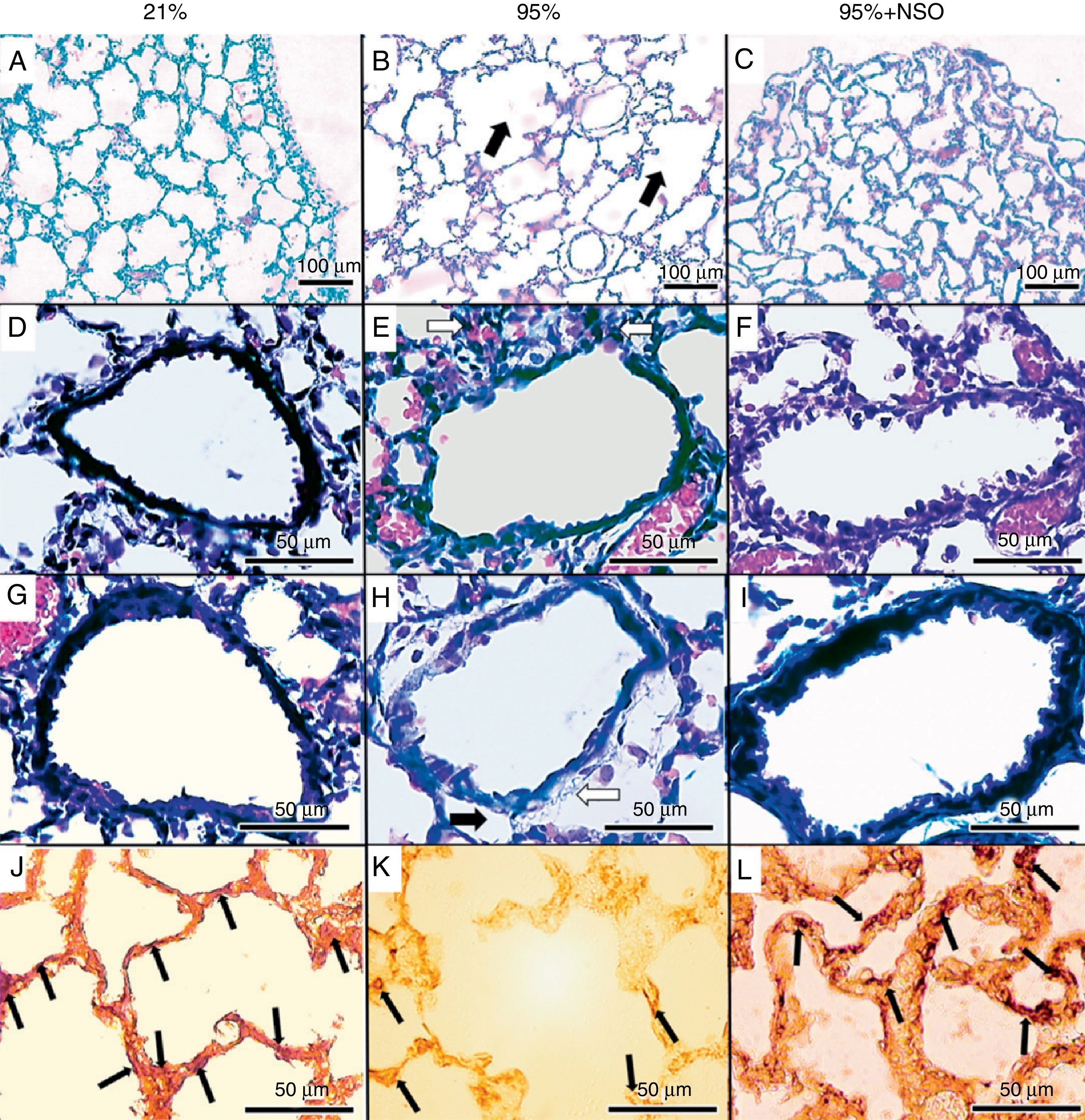
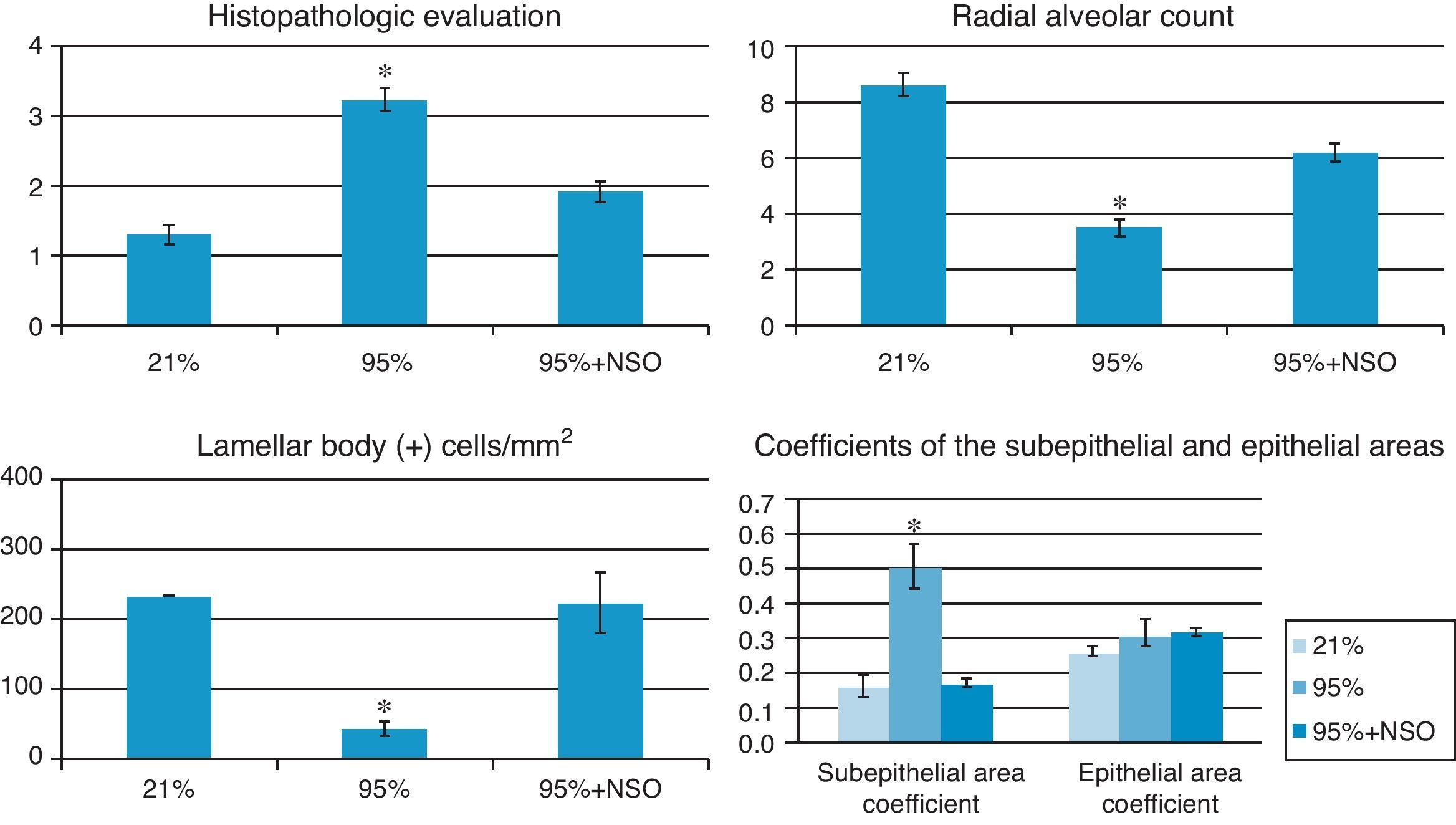
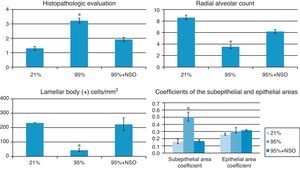
The severity of the lung lesions found was from grade 1 to grade 4 in the histopathologic evaluation (Figs. 1A–C and 2). No alveolar wall thickening or cellular infiltrates were observed in the groups with O2 at 95%+NSO and O2 at 21%. We identified significant changes in the histologic grade and an improvement in the 95% O2+NSO group compared with the group with 95% O2 alone (P<.05). Thus, there was less severe lung lesion in the pups that had been treated with NSO (Fig. 1A–C). The radial alveolar count also showed a recuperation after treatment with NSO (Fig. 2), reflected in a greater number of intact alveoli in this group, with significantly higher radial alveolar counts than those observed in the untreated 95% O2 group (P<.05). In addition, we also observed a better density of lamellar body membrane protein in the NSO group (P<.05). The subepithelial area coefficient demonstrated fibrosis and edema in the 95% O2 group, and this thickening was recuperated with the NSO treatment (P<.05). The epithelium of the intact bronchioles of the 3 groups did not present histological differences, and the epithelial area coefficient showed no significant changes (P>.05).
Hematoxylin-eosin stain (A–C, 100×), Masson's trichrome stain (D–I, 400×) and immunohistochemistry for lamellar body membrane protein (J–L, 400×). There is partial destruction of lung tissue (panel B, black arrows), cell infiltration (panel E, white arrows), edema (black arrow) and fibrosis (white arrow) (panel H) in the 95% O2 group, which is not observed in the 21% O2 or the 95% O2+NSO group. Panels J–L show the immunohistochemistry for the lamellar body membrane protein in each group.
Histogram of the histopathologic evaluation, radial alveolar count, subepithelial/epithelial area coefficients and lamellar body membrane protein counts (cells positive for lamellar bodies/mm2) in each group. The asterisk (*) indicates significant changes (P<.05) compared with the groups with 21% O2 and 95% O2+NSO.
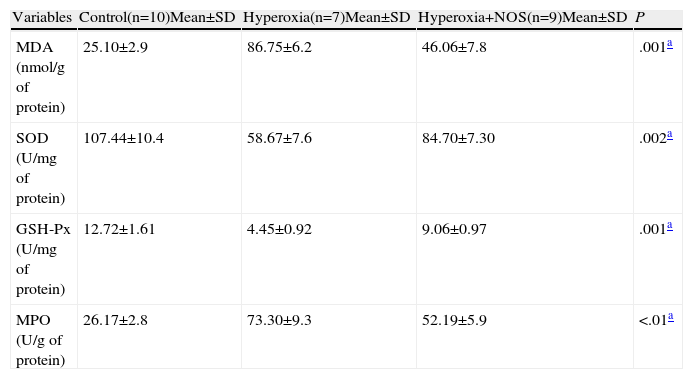
The biochemical analysis was done in samples of lung tissue obtained from the study groups. The levels of GSH-Px and SOD in the tissue were significantly higher in the hyperoxia+NSO group than in the hyperoxia group, and it determined a reduction in oxidative stress (P<.05) (Table 1). However, the levels of MDA and MPO in the tissues of the hyperoxia+NSO group were significantly lower than those of the hyperoxia group, which indicated a reduction of lipid peroxidation due to reactive oxygen species (ROS) and a reduction in the infiltration of neutrophils/inflammation with NSO treatment (Table 1). Furthermore, the levels of MDA and MPO of the control group continued to be lower than those of the hyperoxia+NSO group, in addition to the higher levels of GSH-Px and SOD (P<.05) (Table 1).
Tissue Levels of Malondialdehyde (MDA), Myeloperoxidase (MPO), Superoxide Dismutase (SOD) and Glutathione Peroxidase (GSH-Px) in the Study Groups.
| Variables | Control(n=10)Mean±SD | Hyperoxia(n=7)Mean±SD | Hyperoxia+NOS(n=9)Mean±SD | P |
| MDA (nmol/g of protein) | 25.10±2.9 | 86.75±6.2 | 46.06±7.8 | .001a |
| SOD (U/mg of protein) | 107.44±10.4 | 58.67±7.6 | 84.70±7.30 | .002a |
| GSH-Px (U/mg of protein) | 12.72±1.61 | 4.45±0.92 | 9.06±0.97 | .001a |
| MPO (U/g of protein) | 26.17±2.8 | 73.30±9.3 | 52.19±5.9 | <.01a |
In the present study, we have evaluated the favorable effects of NSO on rat pups with hyperoxia-induced BPD. Our results show that the administration of NSO reduced the pulmonary histopathologic lesion and preserved lung cells. The biochemical analysis identified an increase in antioxidant activities, reduced oxidant levels and a reduction in lipid peroxidation and the infiltration of neutrophils-inflammation in the lung tissues damaged by hyperoxia. As far as we know, this is the first study that examines the effects of NSO in an experimental model of BPD in rats.
BPD has a complex pathogenesis that is not sufficiently understood. In addition to the immaturity of the lungs, it has been suggested that the effects of the oxidative lesion that causes the generation of ROS molecular species play a crucial role in the pathogenesis of BPD.1–4,25–27 ROS are produced by the cellular metabolism of molecular oxygen and the activation of neutrophils and macrophages in the lungs. ROS cause oxidative stress by means of the oxidation of lipids, proteins and DNA.28 Consequently, ROS can produce a tissue lesion that leads to the pathologic alterations that are observed in newborns with BPD.25 In addition, premature newborns show an exclusive vulnerability to oxidative stress, caused by high concentrations of additional oxygen during respiratory support as a consequence of the inadequate and immature antioxidant system at birth as well as the deterioration of upregulation as a response to oxidative stress. Consequently, premature babies present increased risk for developing lung damage induced by ROS.26,29 Antioxidant enzymes like SOD and GSH-Px are important for the elimination of ROS.26 It has been suggested that tissue levels of SOD and GSH-Px may reflect ROS levels.30,31 In addition, MDA, which is a reliable indicator of lipid peroxidation and oxidative stress in tissues, is the product of degradation originated by the oxidation of polyunsaturated fatty acids by ROS activity.32 Thus, ROS can be evaluated indirectly with the determination of MDA and the levels of some antioxidant enzymes like SOD or GSH-Px in tissue.30–32
N. sativa and the oil obtained from it are reservoirs of different bioactive compositions, and they have been demonstrated to reduce tissue injury thanks to their antioxidant activities, especially by reducing oxidative stress through their activity of eliminating radicals.8,33 Moreover, it has been observed that NSO promotes the activity of antioxidant enzymes like GSH-PX and SOD, and it reduces the lipid peroxidation of the biological membranes thanks to their antioxidant properties and elimination of ROS.33 In the present study, we have demonstrated that the increased levels of MDA, as well as the reduction of GSH-PX and SOD levels, determined and supported the lung lesion and lipid peroxidation induced by ROS in the lungs of rats subjected to hyperoxia. However, treatment with NSO produced a decrease in MDA levels as well as an increase in GSH-PX and SOD levels in the rat pups subjected to hyperoxia. These data establish that treatment with NSO reduces oxidative stress and lipid peroxidation, and it promotes endogenous antioxidant enzymes during the hyperoxia process in the lungs.
It is believed that inflammation plays a central role in the pathogenesis of BPD. Premature newborns have a high prevalence of inflammatory disorders such as chorioamnionitis in the neonatal period.1–4,25 Furthermore, hyperoxia can also affect morphogenesis due to its chemotactic effects on inflammatory cells like neutrophils and macrophages. Neutrophils and macrophages produce increased inflammatory response against oxygen exposure, as well as a release of ROS at the place of the inflammation, with the consequent lung lesion. In addition, the existing evidence suggests that the lung lesion of BPD is associated with an increase in the content of neutrophils and chemokines in the lungs.25 Consequently, the number of neutrophils grows persistently both in the interstitium as well as in the airspaces of the lungs of newborns with BPD, and the inhibition of the entrance of neutrophils in the hyperoxic lung promotes postnatal lung growth.34,35 However, many studies have identified that NSO inhibits inflammatory lung responses, peribronchial, alveolar and alveolar wall inflammatory cell infiltration with neutrophils and macrophages, and it has immunomodulatory effects on these cells, in addition to antimicrobial properties against different pathogens.8,10–12,16,17,33 Another important factor that has been suggested to contribute to producing lung damage is human neutrophil elastase (HNE). It has been described that HNE plays an important role in the extracellular proteolytic process and is able to fragment many important proteins like elastin, a crucial protein of the extracellular matrix in the lungs. Nevertheless, NS has been demonstrated to have inhibitory effects on the activity of HNE, and it is believed to be a natural anti-elastase agent that acts against HNE.15 It has also been established that the MPO enzyme is specific for the lysosomes of the neutrophils.36 Thus, the evaluation of the migration and neutrophilic infiltration in the lungs can be done indirectly by determining the activity of MPO in lung tissue.34–37 In the present study, the increased MPO activity indicated that the entrance of neutrophils in the lungs could play a central role in lung injury induced by hyperoxia. Nevertheless, our data showed that the activity of MPO in the lungs was reduced with the NSO treatment during the hyperoxic lesion. Consequently, the results obtained suggest that NSO possesses anti-inflammatory properties and prevented the activation of neutrophils in addition to producing antioxidant effects in rat pups subjected to hyperoxia.
In the present study, pulmonary lesion similar to that observed in newborns with BPD was induced by prolonged hyperoxia and showed pathological alterations in the developing lung tissue, which included the following: (a) decreased alveolarization, demonstrated by a decrease in radial alveolar count (RAC) and thickening of the alveolar walls; (b) reduced expression pattern of lamellar bodies, which indicates severe lung lesion through the reduction of the type II cell count containing lamellar bodies; and (c) a significant increase in fibrosis, which is one of the main histopathologic characteristics of lung injury in BPD.4,25,37–40 However, our results indicated the increase in RAC and reduction in the thickness of the alveolar walls, reduced fibrosis as well as improved lamellar body protein staining pattern density in the group of animals with hyperoxia treated with NSO. All of this is evidence that indicates the prevention of lung injury, with better alveolarization and decreased fibrosis. Consequently, given these results, it would suggest that treatment with NSO protects the lungs against the severe damage caused by lung injury induced by hyperoxia, which is an important contributing factor in the development of BPD. These results could be due to the anti-inflammatory, immunomodulator, cytoprotector and antioxidant properties of NSO, in addition to its activity inhibiting HNE.
In conclusion, the therapeutic objectives in BPD are centered around reducing the persisting lesion to a minimum, reducing inflammation, maintaining adequate oxygenation and promoting pulmonary growth in the developing lungs. Treatment with NSO significantly attenuated the pulmonary lesion by maintaining better alveolarization and reducing fibrosis, inflammation and oxidative stress. Thus, NSO seems to be a promising agent for the protection of lung tissues from severe lesion in BPD. However, new studies will be necessary to determine whether treatment with NSO has favorable effects for protecting the lungs from the severe damage produced by the development of BPD.
FundingThis study was funded by a grant from the GATA Military School of Medicine and the Fatih University Faculty of Medicine Scientific Research Committee.
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: Tayman C, et al. Efectos protectores del aceite de Nigella sativa en la lesión pulmonar inducida por hiperoxia. Arch Bronconeumol. 2012;49:15–21.