Humans spend a considerable amount of their time breathing air inside enclosed spaces in which, due to various sources, there may be contaminants that deteriorate the air quality. This is an important risk factor for the health of the general population.
This review evaluates the contaminants that are present in the air of indoor air spaces, describing the sources that generate them as well as the physiopathological mechanisms and the diseases that they may cause in the respiratory system.
El ser humano pasa una parte considerable de su tiempo respirando el aire de espacios cerrados en los que, por medio de muy diversas fuentes, pueden generarse contaminantes que deterioren su calidad y constituyan un importante factor de riesgo para la salud de la población en general.
En esta revisión se desarrollan los contaminantes presentes en el aire de espacios interiores, describiendo las fuentes que los generan, los mecanismos fisiopatológicos y las enfermedades que pueden producir en el aparato respiratorio.
Activities of daily living require individuals to spend more than 80% of their time indoors (offices, schools, hospitals, daycare, shopping centers, private homes, etc.); therefore, the air quality of such spaces may affect the health of their inhabitants. The WHO has estimated that there are 2 million annual deaths worldwide attributable to indoor air contaminants (IAC),1 and the WHO has also ranked this phenomenon as the tenth avoidable risk factor in importance for the health of the general population.2
The potential contaminants are distinct in origin: those derived from combustion, biological processes and agents, gases and volatile organic compounds (VOC). Contaminants and the pathological processes derived from exposure to them differ according to income level, geographical location and individual cultural determinants. In countries with greater socioeconomic development, IAC are influenced by the architectural design of buildings, outdoor sources of pollution, construction materials and ventilation and air conditioning systems. In less developed countries, the use of biomass as a combustible for cooking or heating in homes is the main source of IAC. Lower respiratory tract infections in children, as well as chronic obstructive pulmonary disease (COPD) and respiratory tract tumors in adults, are the main death-causing pathologies attributable to poor indoor air (IA) quality.3
Our objective is to present a review about IAC describing the substances, sources of pollution and respiratory tract health problems that can be a result. This review will not deal with tobacco smoke exposure or exposures directly related to the work or professional setting, as we understand these to have particularities that would require a specific approach.
Indoor Air QualityEnvironmental quality is defined as the harmony of thermal, acoustic and luminous factors along with the air that is breathed, which should not be a danger for proper health, and the air should be fresh. The IA of a home or building should not contain contaminants at concentrations higher than those that can be detrimental to health or cause malaise among its occupants.4
The chemical composition of IA can be made up of a multitude of substances in small concentrations. The levels of contamination measured in studies done in offices and homes are usually quite lower than that of the permitted limits for industrial settings, but the chemical analysis cannot predict the inhabitants’ perception of the air that they breathe, as a mix of many contaminants, even at low concentrations and combined with humidity and temperature conditions, can make the perception of its quality to be worse. Thus, smell is a useful indicator for assessing IA quality.5 In this direction, the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) has elaborated recommendations for assessing IA, which can be considered good quality when there are no known pollutants in toxic concentrations and a substantial majority (80% or more) of individuals exposed express no discomfort.4
Sources of Contamination and Indoor Air ContaminantsThe factors that affect IA quality are deficiencies in ventilation, outdoor air quality and the presence of indoor contaminant sources.
Ventilation DeficienciesVentilation provides air and should be sufficient to dilute the contaminants to levels below human perception and those considered detrimental for health.
Ventilation may be inadequate due to insufficient air volume, high levels of recirculation, incorrect placement of ventilation points, deficient distribution that leaves certain areas without ventilation and a lack of maintenance or incorrect design of filtering systems.6 In a study carried out in the United States analyzing 97 buildings, it was observed that deficient maintenance of air conditioning systems was associated with increased respiratory, eye and skin symptoms among the occupants.7
In developing countries, biomass fuels are often used for cooking or heating homes, which usually coincides with deficient ventilation of the spaces where the combustion takes place.
Several authors have found a clear relationship between the different ventilation systems (natural, air conditioning or mixed) and IAC. CO2 levels are considered a good parameter for assessing indoor ventilation quality in enclosed settings like day-care centers or schools; levels above the threshold of 1000ppm would imply poorly functioning ventilation systems.8 Of the different ventilation systems, mixed systems are the most effective.9
Outdoor PollutionFrom outside, outdoor contaminants enter into indoor spaces. These include: CO, hydrocarbons and nitrogen oxides, which fundamentally originate from motor vehicle combustion, and sulfur oxides (SO2) and VOC, generated from power plants and industrial processes.
In addition, outdoor ozone that is a secondary contaminant and contaminated IA that is released outside can once again enter through the air conditioning and ventilation systems.
Other contaminants filter through building foundations (gasoline vapors, emanations from sewers and radon). It has been demonstrated that when the concentration of a contaminant increases outdoors, indoor levels also rise, although more slowly.5
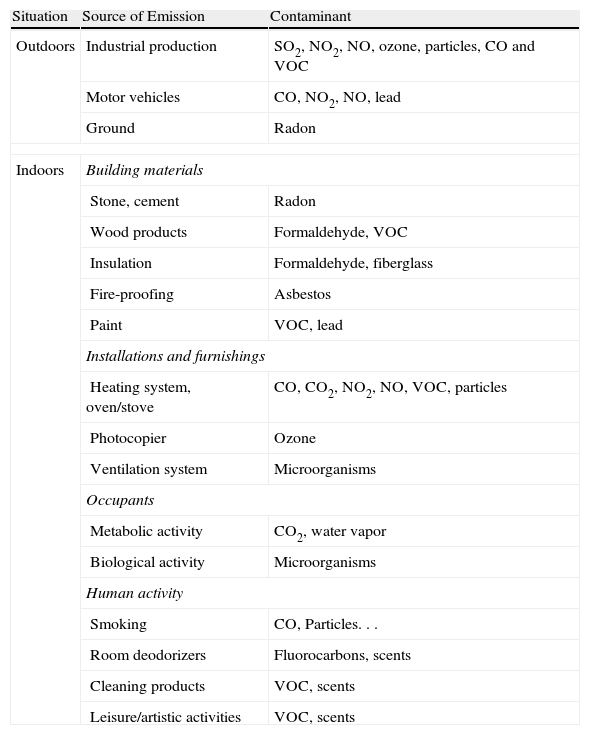
Indoor PollutionActivities that take place, construction materials, furniture, finishings and the use of chemical products all influence IA quality. Table 1 summarizes the main sources of emission and contaminants.
Contaminants and Their Most Common Sources.
| Situation | Source of Emission | Contaminant |
| Outdoors | Industrial production | SO2, NO2, NO, ozone, particles, CO and VOC |
| Motor vehicles | CO, NO2, NO, lead | |
| Ground | Radon | |
| Indoors | Building materials | |
| Stone, cement | Radon | |
| Wood products | Formaldehyde, VOC | |
| Insulation | Formaldehyde, fiberglass | |
| Fire-proofing | Asbestos | |
| Paint | VOC, lead | |
| Installations and furnishings | ||
| Heating system, oven/stove | CO, CO2, NO2, NO, VOC, particles | |
| Photocopier | Ozone | |
| Ventilation system | Microorganisms | |
| Occupants | ||
| Metabolic activity | CO2, water vapor | |
| Biological activity | Microorganisms | |
| Human activity | ||
| Smoking | CO, Particles... | |
| Room deodorizers | Fluorocarbons, scents | |
| Cleaning products | VOC, scents | |
| Leisure/artistic activities | VOC, scents | |
CO, carbon monoxide; CO2, carbon dioxide; VOC, volatile organic compounds; NO, nitrogen monoxide; NO2, nitrogen dioxide.
Sources of chemical contaminants in a building are products of combustion with poor ventilation or deficient maintenance: heaters, ovens, stoves, refrigerators and gas ovens can release different contaminants: CO, NO, NO2, SO2 and particles (PM).
Of special importance is CO, a colorless and odorless gas that is produced by incomplete combustion of carbon substances. Common sources include portable heaters that use kerosene, wood-burning fireplaces, hot water heaters, heaters in poor condition, gas heaters, automobile exhaust and tobacco smoke. At low concentrations, it may cause respiratory symptoms in healthy individuals and exacerbations in patients with chronic cardiopulmonary diseases. It has an asphyxiating effect when bound with hemoglobin, forming carboxyhemoglobin and reducing the oxygen supplied to tissues.10 The main source of IAC in underdeveloped countries is biomass combustion (wood, coal, grass, remains from harvests, excrements, etc.). Although in the modern world these fuel sources have been substituted for cleaner energy sources, 50% of all homes worldwide and 90% of rural homes continue to use biomass as a main source of energy,11 and women and children are especially exposed to its effects. A high number of biomass smoke components are toxic for the respiratory tract: PM, CO, NO2, SO2, formaldehydes and benzopyrene. PM is what has the greatest impact on health. They are reported according to their diameter measured in micrometers (PM10 and PM2.5) and, according to the Environmental Protection Agency (EPA), homes that use BMS have particle levels between 10 and 70 times the environmental levels of the most polluted cities.12
Construction Materials and FurnishingsFiberglass that is used as thermal insulation in air conditioning systems can become easily degraded and break down into particles that can pass through air ducts and reach lung tissue by inhalation. Asbestos used in construction and in insulation materials can emit fibers into IA. Although the WHO has recommended not to use asbestos, it is a material that is still present in older buildings. It can, therefore, be a source of contamination during building maintenance or remodeling and also due to the degradation of the materials that contain it. Those who have the highest risk are workers who participate in asbestos removal.13,14
Furniture, cleaning products, and products used in artistic or artisanal activities are a source of VOC emission, including formaldehyde, benzene or toluene.15 Formaldehyde has been classified as a human carcinogen,16 and its presence is common in wood veneers, panels and agglomerates used in the furniture industry. Aging favors its increased concentration in IA.17 Formaldehyde also appears during the first months of aging of certain varnishes, and therefore its emission may remain over time.
Benzene is a carcinogenic product whose main sources are paints, resins, oils, plastics, detergents and tobacco smoke.
Office-related devices (computers, printers, photocopiers, etc.) and office material (liquid white-out correctors, photography solutions, etc.) are a source of VOC. A high number of computers in an office worsen the subjective sensation of air quality and VOC concentrations.18
Human ActivitiesCleaning and personal hygiene products contain irritating inhalable particles, although they are almost always at low concentrations. In a study published in 2009, there was a very significant number of subjects with reactions to aromatic products19: 19% presented respiratory symptoms, headaches and eye irritation. Insecticides and pesticides also contain organophosphates or hydrocarbons that raise VOC concentrations.20
Physical Contamination: RadonToxic gas leaks through the ground under homes or the water supply can cause IAC, and the main source is the emission of radon radioactive gas.
Radon is a colorless, odorless, tasteless gas with a weight that is 7 times heavier than air that is emitted during the disintegration of uranium in rocks and in the earth. Radon filters through the ground, is diffused in the air and becomes concentrated in closed, poorly ventilated spaces. It is widely distributed, although at low concentrations; continuous inhalation at high concentrations raises the risk for lung cancer (LC).21 The levels that radon may reach in an indoor space depend on the geological characteristics of the terrain, the construction materials used and the ventilation of the building.
Biological ContaminationBacterial endotoxins, fungi and dust mites are considered the main biological contaminants.22 The levels of these contaminants are very variable and can be modified according to the climatological conditions and cleanliness. The accumulation of organic material is a nutrient for fungi and bacteria; thus, wood, paper, paint and carpets can all house microorganisms. Poorly ventilated buildings favor microbial growth. Humidifiers that use recirculated water become contaminated and act as reservoirs. If ventilation systems are not correctly cleaned and disinfected, the occupants may become exposed to different biological agents and also increased transmission of infectious diseases.23 This is characteristic in legionellosis,6 as Legionella spp. multiplies in cooling towers, humidifiers and shower heads, which can disseminate the germ and cause epidemic outbreaks.
Endotoxins are compounds that integrate the external membrane of Gram-negative bacteria, such as enterobacteria or Pseudomonas spp., and they are made up of proteins, lipids and lipopolysaccharides. The latter are responsible for the majority of the biological effects of endotoxins. The individual immunological response will depend on the dose of the exposure, the period of exposure (childhood, adulthood) and individual genetic predisposition.24
Endotoxin levels are greater in the spring-summer months and they descend in fall-winter.25 The effects on health are variable; thus, toxic syndrome due to exposure to organic dust is acute and similar to hypersensitivity pneumonitis but caused by the direct effect of endotoxins, without the mediation of any immunological mechanism. Chronic exposure can provoke different symptoms, such as fever, headache, throat irritation, cough, wheezing and thoracic oppression.26 There are several studies that demonstrate that exposure to endotoxins cause a fall in FVC and in FEV1; nonetheless, these were done in work settings, with intense occupational exposure. Contrarily, other studies show that exposure to endotoxins at early ages seems to have a protector effect against the development of atopic diseases by means of a stimulus of the Th-1 response versus Th-2.27
Most antigens in IA come from dust mites and fungi, especially in bed linens, carpets and upholstered furniture. It has been demonstrated that the exposure to dust mites is a fundamental factor in the sensitization and the development of atopic pathology. A classic study by Platts-Mills et al.28 established a threshold for sensitization as the presence of 2μg/g of dust.
Exposure to fungi can cause pathology by several mechanisms: immunological (allergic), infection and irritating effects caused by the exposure to several volatile compositions (mycotoxins, 1,3-βd glucan).29 The most common are Penicillium, Cladosporium, Alternaria, Aspergillus and Eurotium, among others. Humidity and heat favor their growth and can be found in showers or basements with high levels of humidity, and also in the water found in humidifiers or their filters.
Lastly, emphasis must be given to the importance of pollen. It generally comes from outdoor plants, and indoor pollen concentrations are usually low; however, concentrations may become high in poorly ventilated buildings.30 Furthermore, an interaction has been observed between environmental contaminants and pollens that promote allergenicity.31
PhysiopathologySeveral physiopathological mechanisms have been described by which different contaminants produce their effect.
Smoke generated during the combustion of biomass and carbon produces inhalable particles and organic compositions (benzopyrene, formaldehyde or benzene32) that can alter defense mechanisms of the lungs, such as mucociliary clearance or the function of macrophages.33,34 The particles cause direct bronchial irritation and inflammation, while increasing oxidative stress or conditioning DNA alterations.35–37 It has also been observed that the thickening of the vascular intima is greater in those exposed to BMS smoke than in smokers.38 Likewise, carbon monoxide may be produced, which binds with hemoglobin, generating carboxyhemoglobin and the consequent reduction in oxygen-transporting capacity.37
NO2 causes alterations in airway caliber and the viscoelastic properties of the lungs, while deteriorating the gas exchange.39
Exposure to fungi can induce cytotoxic and immunosuppressant inflammatory responses.40
VOC cause direct irritation of the mucosa, inflammation and obstruction of the airway, and induce oxidative stress. They also promote sensitization mechanisms by means of a synergist effect, thus causing an allergic reaction with a smaller quantity of allergen, as well as the reduction in s-nitrosoglutathione, which is an endogenous bronchodilator.41,42
Radon decays into solid radioactive elements, like polonium, which release alpha particles, a form of high-energy radiation that can deteriorate human DNA. The energy of these particles is regardless of radon concentrations, so that even at low concentrations it can be harmful, and therefore the toxicity of radon does not require a threshold level.32
Correlation Between Contaminants and Respiratory DiseaseIAC are related with multiple respiratory diseases: some with a high level of evidence (as in the case of asthma, COPD, respiratory infections, rhinitis or LC), and in others with a lesser degree of evidence but with data that suggest a certain solidity (such as pulmonary tuberculosis, hypersensitivity pneumonitis or other diffuse interstitial lung diseases).33,37
InfectionsThe relationship between respiratory infections and IAC has been well established. The presence of humidity or fungi has been consistently associated with the appearance of infections (OR: 1.50; 95% CI: 1.32–1.70) both in children and in adults.40 Exposure to biomass smoke raises the risk for respiratory infections in children (OR: 3.53; 95% CI: 1.93–6.43),43 and Dherani et al., in a recent meta-analysis, observed an increase of 80% in the risk for pneumonia in children under the age of 5 related with IAC due to the use of solid combustibles (OR: 1.79; 95% CI: 1.26–2.21).44 Moreover, the risk is dependent of dosage and increases with high concentrations of contaminants.37
A recent intervention study in Guatemala analyzed exposure in children who were under the age of 4 months and followed until the age of 18 months. In the home of one group, stoves with chimneys were installed, while the other group maintained the traditional way of cooking over an open fire. There was an observed significant reduction in the incidence of severe pneumonia in the intervention group.34 The greatest reduction in incidence was seen in cases where there was no infection due to respiratory syncytial virus. This could indicate that IAC favor bacterial pneumonia.34
Chronic Bronchitis and Chronic Obstructive Pulmonary DiseaseSmoking is the fundamental etiologic agent in COPD, but the population attributable fraction for tobacco use is variable, ranging between 9.7% and 97.9%, so that other agents may also cause this disease.45
The characteristics of COPD related to domestic exposure to biomass combustion are different than those caused by smoking: there is a greater component of fibrosis, anthracosis and hyperplasia of the intima of the pulmonary arteries.33
The typical patient with COPD due to exposure to biomass fuel is usually an older woman, non-smoker, with a history of exposure, spirometry with mild or moderate airflow limitation and slightly altered diffusion capacity.33
Several systematic reviews and meta-analyses have analyzed the relationship between the exposure to biomass smoke and the presence of chronic bronchial pathology, demonstrating significant increases both in the prevalence of COPD (OR between 2.4 and 2.8) as well as chronic bronchitis (OR between 2.3 and 2.6) in the exposed population.43,46,47
Other contaminants have been related with the prognosis of COPD. A recent study has observed that for every 100Bq/m3 of increased radon, mortality due to COPD increased 13% (Hazard ratio: 1.13; 95% CI: 1.05–1.21).48
Lung CancerAround 15% of LC cases are present in non-smokers.32 Several IAC are considered carcinogens.
Radon was the first environmental agent to be identified as a cause of cancer, and asbestos is clearly recognized as a carcinogenic agent. Biomass combustion smoke has consistently been associated with increased risk for LC, as has also been smoke from food (especially frying with oil at high temperatures), although evidence is limited. In women who are never-smokers, most studies find some association between the preparation of fried foods and LC, but other studies find no correlation.32
In a study in a Mexican population, 38.7% of the cases of LC occurred in non-smoker patients with long-term home exposure to biomass smoke, with no exposure to other carcinogens.49 In women from India, it was observed that the use of biomass fuel increased the risk for LC (OR: 3.59; 95% CI: 1.08–11.97).50 It has recently been observed in sputum samples that there is deterioration in the DNA of individuals exposed to biomass smoke compared to those who use liquid gas for cooking, and it seems to be at least partially mediated by oxidative stress generated by the inhalation of particles and benzene.51 There has also been a demonstrated carcinogen effect of coal smoke, which significantly increases the risk for LC in exposed people (OR: 2.55; 95% CI: 1.58–4.10).37
The Cancer Prevention Study-II observed that exposure to radon levels higher than 148Bq/m3 (considered the tolerance threshold by the EPA) represented an increase of 34% (95% CI: 7–68) in the risk of dying from LC.52 Likewise, in a case–control study done in Galicia, Ruano-Ravina et al.53 reported an increased risk (relative risk: 6.6; 95% CI: 1.2–38) for LC with exposure higher than 148Bq/m.3 Studies in other populations show similar findings and relate this gas with 3.3% of deaths due to LC in the United Kingdom, and between 18% and 28% in Portugal.54,55
Bronchial AsthmaSome studies have shown that exposure to biomass smoke increases asthma prevalence and severity,33,37,56 although a recent meta-analysis questions these results.43 In the previously mentioned intervention in Guatemala,34 providing stoves with chimneys showed favorable results in the intervention group, as in the follow-up until 18 months the chimney group had less respiratory symptoms (OR: 0.70; 95% CI: 0.50–0.97).57 The divergence between the results of the different studies could depend on the different methodologies used to measure disease or exposure, with differences between the populations studied or covariables considered.
The increased level of NO2 in IA has been more frequently related with nocturnal cough, wheezing and use of bronchodilator medication, both in children as well as adults. The effect seems to be greater in non-atopic patients, which suggests a different inflammatory mechanism than allergy.39,58,59
Environmental contamination by fungi has also been related with the prevalence of asthma, with poorer control of the disease or with more exacerbations, evaluated both qualitatively with the presence of water stains as well as objectively by measuring levels of some fungi components like ergosterol or B-D-glucan.40,60–62 Associations seem to be consistent with a dose-response relationship, and therefore exposure to greater levels of fungi condition more expression of the disease.40
VOC easily penetrate the airway due to their capacity for presenting in the form of vapors or gases. A recent systematic review seems to confirm an increase in the prevalence of asthma in children of 3% for every 10μg/m3 of increase in formaldehyde levels.42 A case–control study with children demonstrated that the exposure to other VOC like acetaldehyde or toluene significantly increases the prevalence of asthma. The effect seems different depending on whether the residence is urban or rural or on the season of the year.63 An increase has also been detected in the prevalence of asthma and a decline in the control of the disease in individuals exposed to cleaning products.64
Particles, PM10 as well as PM2.5, have been associated with the increase in asthma symptoms and with greater use of rescue medication.65,66
As for the presence of animals in the home, there seem to be differences depending on the type of animal. Greater exposure to cockroaches, rats, birds or dogs seems to be related with higher asthma prevalence and symptoms.60,67–69 However, exposure to cats does not seem to increase asthma symptoms, and a recent meta-analysis has even observed a protector effect for asthma from exposure to this animal.70,71
OthersFor other diseases, the evidence is less consistent. A greater prevalence of rhinitis has been observed with exposure to humidity, fungi and some VOC,40,41,64 and increased incidence of tuberculosis has been reported with exposure to biomass combustion.33,37,72
In addition, there have been reports of cases of hypersensitivity pneumonitis related with certain fungi73–75 or ventilation systems.76 It has also been suggested that biomass smoke exposure may produce lung fibrosis.38
Potential Areas of ResearchDespite the importance of IA quality for health, the information available about some aspects is still scarce. Research programs in this area could include:
- •
Standardization of exposure monitoring and measuring systems.
- •
Possible dose-response relationships that could identify safe exposure levels.
- •
Intervention studies assessing the health benefits of reduced exposure.
- •
Short- and long-term socioeconomic impact of exposure and interventions to reduce it.
- •
Identification of physiopathological mechanisms and the population most susceptible to certain exposures.
IAC are a risk factor for multiple respiratory tract diseases. Many sources are able to generate indoor contaminants, and there is a direct correlation between income levels and geographic and cultural determinants. Therefore, different actions adapted to each specific setting are required. From the standpoint of a pulmonologist, it is important to recognize the problem in each patient and stimulate lines of research in this field of knowledge.
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: Carazo Fernández L, et al. Contaminación del aire interior y su impacto en la patología respiratoria. Arch Bronconeumol. 2012;49:22–7.