This Executive Summary of the Global Strategy for the Diagnosis, Management, and Prevention of COPD (GOLD) 2017 Report focuses primarily on the revised and novel parts of the document. The most significant changes include: 1) the assessment of COPD has been refined to separate the spirometric assessment from symptom evaluation. ABCD groups are now proposed to be derived exclusively from patient symptoms and their history of exacerbations; 2) for each of the groups A to D, escalation strategies for pharmacological treatments are proposed; 3) the concept of de-escalation of therapy is introduced in the treatment assessment scheme; 4) nonpharmacologic therapies are comprehensively presented and; 5) the importance of comorbid conditions in managing COPD is reviewed.
Este resumen ejecutivo del Informe de 2017 de la Global Strategy for the Diagnosis, Management, and Prevention of COPD (GOLD) se basa primordialmente en las modificaciones y novedades del informe anterior. Los cambios más destacados incluyen: a) se diferencia la exploración espirométrica de la de los síntomas para la evaluación de la enfermedad pulmonar obstructiva crónica (EPOC); de este modo, los grupos ABCD se refieren exclusivamente a síntomas y antecedentes de exacerbaciones de los pacientes; b) se optimiza la estrategia terapéutica farmacológica en cada uno de los cuatro grupos; c) se propone una reducción escalonada de la medicación en el apartado terapéutico; d) se detalla más extensamente el tratamiento no farmacológico; y, f) se repasa la importancia de las diferentes co-morbilidades en lo que respecta al tratamiento de la EPOC.
This Executive Summary of the Global Strategy for the Diagnosis, Management, and Prevention of COPD (GOLD) 2017 Report is based on peer-reviewed publications to October 2016.
Levels of evidence are assigned to evidence-based recommendations where appropriate. Categories used to grade the levels of evidence are provided in Table S1 in the Supplementary Appendix.
Definition and Factors That Influence COPD Development and ProgressionKey Points[TS: Set all “Key Points”] boxes as they were in original GOLD (http://www.atsjournals.org/doi/pdf/10.1164/rccm.201204-0596PP).]
- •
COPD is a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.
- •
Dyspnea, cough and/or sputum production are the most frequent symptoms; symptoms are commonly under-reported by patients.
- •
Tobacco smoking is the main risk exposure for COPD, but environmental exposures like biomass fuel exposure and air pollution may contribute. Besides exposures, host factors (genetic abnormalities, abnormal lung development and accelerated aging) predispose individuals to develop COPD.
- •
COPD may be punctuated by acute worsening of respiratory symptoms, called exacerbations.
- •
In most patients, COPD is associated with significant concomitant chronic diseases, which increase morbidity and mortality.
COPD is a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.
The chronic airflow limitation that characterizes COPD is caused by a mixture of small airways disease (e.g., obstructive bronchiolitis) and parenchymal destruction (emphysema), the relative contributions of which vary from person to person. Chronic inflammation causes structural changes, small airways narrowing and destruction of lung parenchyma. A loss of small airways may contribute to airflow limitation and mucociliary dysfunction, a characteristic feature of the disease.
Chronic respiratory symptoms may precede the development of airflow limitation and be associated with acute respiratory events.1 Chronic respiratory symptoms may exist in individuals with normal spirometry1,2 and a significant number of smokers without airflow limitation have structural evidence of lung disease manifested by the presence of emphysema, airway wall thickening and gas trapping.1,2
Factors That Influence Disease Development and ProgressionAlthough cigarette smoking is the most well studied COPD risk factor, epidemiologic studies demonstrate that non-smokers may also develop chronic airflow limitation.3 Compared to smokers with COPD, never smokers with chronic airflow limitation have fewer symptoms, milder disease and a lower burden of systemic inflammation.4 Never smokers with chronic airflow limitation do not have an increased risk of lung cancer, or cardiovascular comorbidities; however, they have an increased risk of pneumonia and mortality from respiratory failure.4
Processes occurring during gestation, birth, and exposures during childhood and adolescence affect lung growth.5,6 Reduced maximal attained lung function (as measured by spirometry) may identify individuals at increased risk for COPD.2,7 Factors in early life termed “childhood disadvantage factors” are as important as heavy smoking in predicting lung function in adult life.8 An examination of three different longitudinal cohorts found that approximately 50% of patients developed COPD due to an accelerated decline in FEV1; the other 50% developed COPD due to abnormal lung growth and development.
Cigarette smokers have a higher prevalence of respiratory symptoms and lung function abnormalities, a greater annual rate of decline in FEV1, and a greater COPD mortality rate than non-smokers.9 Other types of tobacco (e.g., pipe, cigar, water pipe)10–12 and marijuana13 are also risk factors for COPD. Passive exposure to cigarette smoke, also known as environmental tobacco smoke (ETS), may also contribute to respiratory symptoms and COPD14 by increasing the lung's total burden of inhaled particles and gases. Smoking during pregnancy may pose a risk for the fetus, by affecting in utero lung growth and development, and possibly priming the immune system.15
Occupational exposures, including organic and inorganic dusts, chemical agents and fumes, are under-appreciated risk factors for COPD development.16,17
Wood, animal dung, crop residues, and coal, typically burned in open fires or poorly functioning stoves, may lead to indoor air pollution.18 Indoor pollution from biomass cooking and heating, in poorly ventilated dwellings, is a risk for COPD.19–21
Asthma may be a risk for the development of chronic airflow limitation and COPD.22
Airway hyper-responsiveness can exist without a clinical diagnosis of asthma and is an independent predictor of COPD and respiratory mortality in population studies23,24 and may indicate a risk for excessive lung function decline in mild COPD.25
A history of severe childhood respiratory infection is associated with reduced lung function and increased respiratory symptoms in adulthood.26 HIV infection accelerates the onset of smoking-related emphysema and COPD27; tuberculosis has also been identified as a risk for COPD as well as a potential comorbidity.28–30
Diagnosis and Initial AssessmentKey Points
- •
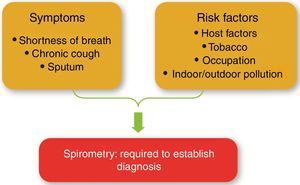
COPD should be considered in any patient with dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors.
- •
Spirometry is required to make the diagnosis; a post-bronchodilator FEV1/FVC<0.70 confirms the presence of persistent airflow limitation.
- •
The goals of COPD assessment are to determine the level of airflow limitation, the impact of disease on the patient's health status, and the risk of future events (such as exacerbations, hospital admissions, or death) to guide therapy.
- •
Concomitant chronic diseases occur frequently in COPD patients and should be treated because they can independently affect mortality and hospitalizations.
COPD should be considered in any patient with dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease (Fig. 1 and Table 1). Spirometry is required to make the diagnosis in this clinical context31; a post-bronchodilator FEV1/FVC<0.70 confirms the presence of persistent airflow limitation and identifies the presence of COPD in patients with appropriate symptoms and predisposing risks.
Key indicators for considering a dagnosis of COPD.
| Consider COPD, and perform spirometry, if any of these indicators are present in an individual over age 40. These indicators are not diagnostic themselves, but the presence of multiple key indicators increases the probability of a diagnosis of COPD. Spirometry is required to establish a diagnosis of COPD. | |
| Dyspnea that is: | Progressive over time. |
| Characteristically worse with exercise. | |
| Persistent. | |
| Chronic cough: | May be intermittent and may be unproductive. |
| Recurrent wheeze. | |
| Chronic sputum production: | With any pattern. |
| Recurrent lower respiratory tract infections | |
| History of risk factors: | Host factors (such as genetic factors, congenital/developmental abnormalities etc.). |
| Tobacco smoke. | |
| Smoke from home cooking and heating fuels. | |
| Occupational dusts, vapors, fumes, gases and other chemicals. | |
| Family history of COPD and/or childhood factors: | For example low birthweight, childhood respiratory infections. |
Chronic and progressive dyspnea is the most characteristic symptom of COPD.
Dyspnea. Dyspnea is a major cause of the disability and anxiety in COPD.32 The terms used to describe dyspnea vary individually and culturally.33
Cough. Chronic cough is often the first symptom of COPD and frequently discounted by the patient as a consequence of smoking and/or environmental exposures.
Sputum production. Regular sputum production>3 months in 2 consecutive years is the classical definition of chronic bronchitis34; an arbitrary definition that does not reflect the range of sputum production reported in COPD. Patients producing large volumes of sputum may have underlying bronchiectasis.
Wheezing and chest tightness. Wheezing and chest tightness may vary between days, and throughout a single day.
Additional features in severe disease. Fatigue, weight loss and anorexia are common in patients with more severe forms of COPD.35,36
Medical HistoryA detailed medical history of any patient who is known, or suspected, to have COPD should include:
- •
Exposure to risk factors, such as smoking and occupational or environmental exposures.
- •
Past medical history, including asthma, allergy, sinusitis, or nasal polyps; respiratory infections in childhood; other chronic respiratory and non-respiratory diseases.
- •
Family history of COPD or other chronic respiratory diseases.
- •
Pattern of symptom development: age of onset, type of symptom, more frequent or prolonged “winter colds,” and social restriction.
- •
History of exacerbations or previous hospitalizations for a respiratory disorder.
- •
Presence of comorbidities, such as heart disease, osteoporosis, musculoskeletal disorders, and malignancies.
- •
Impact of disease on patient's life, including limitation of activity, missed work and economic impact, and feelings of depression or anxiety.
- •
Social and family support available to the patient.
- •
Possibilities for reducing risk factors, especially smoking cessation.
Although important for general health, a physical examination is rarely diagnostic in COPD. Physical signs of airflow limitation/hyperinflation are usually not identifiable until significantly impaired lung function is present.37,38
SpirometrySpirometry is the most reproducible and objective measurement of airflow limitation. It is a noninvasive and readily available test. Good quality spirometry is possible in any healthcare setting; all healthcare workers who care for COPD patients should have access to spirometry.
A post-bronchodilator fixed ratio of FEV1/FVC<0.70 is the spirometric criterion for airflow limitation. This criterion is simple and independent of reference values and has been used in numerous clinical trials. However, it may result in more frequent diagnosis of COPD in the elderly,39,40 and less frequent diagnosis in adults<45 years,40 especially in mild disease, compared to a cut-off based on the lower limit of normal (LLN) values for FEV1/FVC. Several limitations occur with using LLN as the diagnostic criterion for spirometric obstruction: 1) LLN values are dependent on the choice of reference equations that use post-bronchodilator FEV1, 2) there are no longitudinal studies that validate using the LLN, and 3) studies using LLN in populations where smoking is not the major cause of COPD are lacking.
Normal spirometry may be defined by a new approach from the Global Lung Initiative (GLI).41,42 Using GLI equations, z scores were calculated for FEV1, FVC, and FEV1/FVC and compared to fixed ratio data. The findings suggest that among adults with GLI-defined normal spirometry, the use of a fixed ratio may misclassify individuals as having respiratory impairment. These findings await additional study in other cohorts.
The risk of misdiagnosis and over-treatment using the fixed ratio as a diagnostic criterion is limited since spirometry is only one parameter used to establish the clinical diagnosis of COPD. GOLD favors using the fixed ratio over LLN since diagnostic simplicity and consistency are crucial for the busy clinician.
Assessing the degree of reversibility of airflow limitation (e.g., measuring FEV1 before and after bronchodilator or corticosteroids) to make therapeutic decisions is not recommended43 since it does not aid the diagnosis of COPD, differentiate COPD from asthma, or predict the long-term response to treatment.44
In asymptomatic individuals without exposures to tobacco or other noxious stimuli, screening spirometry is not indicated. However, in those with symptoms and/or risk factors (e.g.,>20 pack-years of smoking or recurrent chest infections), the diagnostic yield for COPD is relatively high and spirometry should be considered.45,46 GOLD advocates active case finding45,47 i.e., performing spirometry in patients with symptoms and/or risk factors, but not routine screening spirometry in asymptomatic individuals without COPD risk factors.
AssessmentThe goals of COPD assessment to guide therapy are 1) to determine the level of airflow limitation; 2) to define its impact on the patient's health status and; 3) identify the risk of future events (such as exacerbations, hospital admissions or death).
To achieve these goals, COPD assessment must consider separately the following aspects of the disease:
- •
Presence and severity of the spirometric abnormality
- •
Current nature and magnitude of symptoms
- •
History/future risk of exacerbations
- •
Presence of comorbidities
Spirometry should be performed after administration of an adequate dose of at least one short-acting inhaled bronchodilator in order to minimize variability.
The role of spirometry for the diagnosis, assessment and follow-up of COPD is summarized in Table 2.
Role of spirometry.
| • Diagnosis |
| • Assessment of severity of airflow obstruction (for prognosis) |
| • Follow-up assessment |
| ○ Therapeutic decisions. |
| ■ Pharmacological in selected circumstances (e.g., discrepancy between spirometry and level of symptoms). |
| ■ Consider alternative diagnoses when symptoms are disproportionate to degree of airflow obstruction. |
| ■ Non-pharmacological (e.g., interventional procedures). |
| ○ Identification of rapid decline. |
COPD was previously viewed as a disease largely characterized by breathlessness. A simple measure of breathlessness such as the Modified British Medical Research Council (mMRC) Questionnaire48 was considered adequate for assessment of symptoms49–51 However, COPD impacts patients well beyond dyspnea.52 For this reason, a comprehensive assessment of symptoms is recommended. The most comprehensive disease-specific health status questionnaires include the Chronic Respiratory Questionnaire (CRQ)53 and St. George's Respiratory Questionnaire (SGRQ).54 These are too complex to use in clinical practice, but shorter measures e.g., the COPD Assessment Test (CATTM) are suitable.
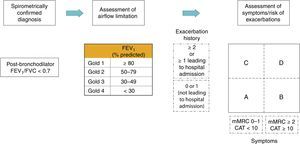
Choice of thresholdsSGRQ scores<25 are uncommon in COPD patients55 and scores ≥ 25 are very uncommon in healthy persons.56,57 The equivalent cut-point for the CATTM is 10.58 A mMRC threshold of ≥ 2 is used to separate “less breathlessness” from “more breathlessness”.
Assessment of exacerbation riskThe best predictor of frequent exacerbations (defined as ≥ 2 exacerbations per year) is a history of earlier treated events.59 Hospitalization for a COPD exacerbation has a poor prognosis and an increased risk of death.60
Blood eosinophil count. Post-hoc analysis of two clinical trials in COPD patients with an exacerbation history showed that higher blood eosinophil counts may predict increased exacerbation rates in patients treated with long acting beta agonists (LABA) (without inhaled corticosteroid, ICS).61,62 The treatment effect of ICS/LABA versus LABA on exacerbations was greater in patients with higher blood eosinophil counts. These findings suggest that blood eosinophil counts are 1) a biomarker of exacerbation risk in patients with a history of exacerbations and 2) can predict the effects of ICS on exacerbation prevention. Prospective trials are required to validate the use of blood eosinophil counts to predict ICS effects, to determine a cut-off threshold for blood eosinophils that predicts exacerbation risk, and to clarify blood eosinophil cut-off values that could be used in clinical practice.
Assessment of concomitant chronic diseases (comorbidities)Patients with COPD often have important concomitant chronic illnesses as COPD represents an important component of multimorbidity particularly in the elderly.60,63–65
Revised combined COPD assessmentThe “ABCD” assessment tool of the 2011 GOLD Report was a major step forward from the simple spirometric grading system of earlier GOLD Reports because it incorporated patient-reported outcomes and highlighted the importance of exacerbation prevention in COPD management. However, there were important limitations. ABCD assessment performed no better than spirometric grades for mortality prediction, or other important health outcomes.66–68 Moreover, group “D” outcomes were modified by two parameters: lung function and/or exacerbation history, which caused confusion.69 To address these concerns, the 2017 GOLD Report provides a refinement of the ABCD assessment that separates spirometric grades from “ABCD” groupings. For some therapy recommendations, especially pharmacologic treatments, ABCD groups are derived exclusively from patient symptoms and their exacerbation history. However, spirometry, in conjunction with patient symptoms and exacerbation history, remains vital for the diagnosis, prognostication and consideration of other important therapeutic approaches, especially non-pharmacological therapies. This new approach to assessment is illustrated in Fig. 2.
In the refined assessment scheme, patients should undergo spirometry to determine the severity of airflow limitation (i.e., spirometric grade). They should also undergo assessment of either dyspnea using mMRC or symptoms using CATTM. Finally, their history of exacerbations (including prior hospitalizations) should be recorded.
The number provides information regarding severity of airflow limitation (spirometric grades 1 to 4) while the letter (groups A to D) provides information regarding symptom burden and risk of exacerbation. FEV1 is a very important parameter at the population-level in the prediction of important clinical outcomes such as mortality and hospitalizations or prompting consideration for non-pharmacologic therapies such as lung reduction or lung transplantation. However, at the individual patient level, FEV1 loses precision and thus cannot be used alone to determine all therapeutic options. Furthermore, in some circumstances, such as during hospitalization or urgent presentation to the clinic or emergency room, the ability to assess patients based on symptoms and exacerbation history, independent of the spirometric value, allows clinicians to initiate a treatment plan based on the revised ABCD scheme. This approach acknowledges the limitations of FEV1 in making treatment decisions for individualized patient care and highlights the importance of patient symptoms and exacerbation risks in guiding therapies in COPD. The separation of airflow limitation from clinical parameters makes it clearer what is being evaluated and ranked. This should facilitate more precise treatment recommendations based on parameters that are driving the patient's symptoms at any given time.
Example. Consider two patients - both patients with FEV1<30% of predicted, CAT scores of 18 and one with no exacerbations in the past year, and the other with three exacerbations in the past year. Both would have been labelled GOLD D in the prior classification scheme. However, with the new proposed scheme, the subject with 3 exacerbations in the past year would be labelled GOLD grade 4, group D. Individual decisions on pharmacotherapeutic approaches would use the recommendations based on the ABCD assessment to treat the patient's major problem at this time, i.e., persistent exacerbations. The other patient, who has had no exacerbations, would be classified as GOLD grade 4, group B. In such patients —besides pharmacotherapy and rehabilitation —lung reduction, lung transplantation or bullectomy may be important therapeutic considerations given their symptom burden and level of spirometric limitation.
Alpha-1 antitrypsin deficiencyThe World Health Organization recommends that all patients with a diagnosis of COPD be screened once for alpha-1 antitrypsin deficiency.70 A low concentration (< 20% normal) is suggestive of homozygous deficiency. Family members should be screened and together with the patient referred to specialist centres for advice and management.
Additional investigationsIn order to rule out other concomitant disease contributing to respiratory symptoms, or in cases where patients do not respond to the treatment plan as expected, additional testing may be required. Thoracic imaging (chest x-ray, chest CT); assessment of lung volumes and/or diffusion capacity, oximetry and arterial blood gas measurement and exercise testing and assessment of physical activity should be considered.
Composite scores. The BODE (Body mass index, Obstruction, Dyspnea, and Exercise) method gives a composite score that is a better predictor of subsequent survival than any single component.71 Simpler alternatives that do not include exercise testing need validation to confirm suitability for routine clinical use.72,73
Differential diagnoses. In some patients, features of asthma and COPD may coexist. The terms Asthma-COPD Overlap Syndrome (ACOS) or Asthma-COPD Overlap (ACO) acknowledge the overlap of these two common disorders causing chronic airflow limitation rather than a distinct syndrome. Most other potential differential diagnoses are easier to distinguish from COPD.
Other considerations. Some patients without evidence of airflow limitation have evidence of structural lung disease on chest imaging (emphysema, gas trapping, airway wall thickening). Such patients may report exacerbations of respiratory symptoms or even require treatment with respiratory medications on a chronic basis. Whether these patients have acute or chronic bronchitis, a persistent form of asthma or an earlier presentation of what will become COPD as it is currently defined, is unclear and requires further study.
Prevention and Maintenance TherapyKey Points
- •
Smoking cessation is key. Pharmacotherapy and nicotine replacement increase long-term smoking abstinence rates.
- •
The effectiveness and safety of e-cigarettes as a smoking cessation aid is uncertain.
- •
Pharmacologic therapy can reduce COPD symptoms, reduce the frequency and severity of exacerbations, and improve health status and exercise tolerance.
- •
Each pharmacologic treatment regimen should be individualized and guided by the severity of symptoms, risk of exacerbations, side-effects, comorbidities, drug availability and cost, and the patient's response, preference and ability to use various drug delivery devices.
- •
Inhaler technique needs to be assessed regularly.
- •
Influenza and pneumococcal vaccinations decrease the incidence of lower respiratory tract infections.
- •
Pulmonary rehabilitation improves symptoms, quality of life, and physical and emotional participation in everyday activities.
- •
In patients with severe resting chronic hypoxemia, long-term oxygen therapy improves survival.
- •
In patients with stable COPD and resting or exercise-induced moderate desaturation, long-term oxygen treatment should not be prescribed routinely, however, individual patient factors should be considered.
- •
In patients with severe chronic hypercapnia and a history of hospitalization for acute respiratory failure, long-term non-invasive ventilation may decrease mortality and prevent re-hospitalization.
- •
In select patients with advanced emphysema refractory to optimized medical care, surgical or bronchoscopic interventional treatments may be beneficial.
- •
Palliative approaches are effective in controlling symptoms in advanced COPD.
Smoking cessation influences the natural history of COPD. If effective resources and time are dedicated to smoking cessation, long-term quit success rates of up to 25% can be achieved.74
Nicotine replacement products. Nicotine replacement therapy increases long-term smoking abstinence rates75–77 and is more effective than placebo. E-cigarettes are increasingly used as a form of nicotine replacement therapy, although their efficacy remains controversial.78–82
Pharmacologic products. Varenicline,83 bupropion,84 and nortriptyline85 increase long-term quit rates,85 but should be used as part of an interventional program rather than as a sole intervention.
Smoking cessation programs. A five-step program for intervention86,87 provides a framework to guide healthcare providers to help patients stop smoking.77,86,88 Counseling delivered by health professionals significantly increases quit rates over self-initiated strategies.89 The combination of pharmacotherapy and behavioral support increases smoking cessation rates.90
VaccinationsInfluenza vaccine and Pneumococcal vaccinesInfluenza vaccination reduces serious illness,91 death,92–95 the risk of ischemic heart disease96 and the total number of exacerbations.92 Vaccines containing either killed or live inactivated viruses are recommended97 as they are more effective in elderly patients with COPD.98
Pneumococcal vaccinations, PCV13 and PPSV23, are recommended for all patients ≥ 65 years of age (see Table S2 in the Supplementary Appendix).
Pharmacologic Therapy for Stable COPDOverview of medicationsPharmacologic therapy for COPD reduces symptoms, the frequency and severity of exacerbations, and improves exercise tolerance and health status. No existing medication modifies the long-term decline in lung function.99–103 The classes of medications used to treat COPD are shown in Table S3 of the Supplementary Appendix. The choice within each class depends on the availability and cost of medication and favorable clinical response balanced against side effects. Each treatment regimen needs to be individualized as the relationship between severity of symptoms, airflow limitation, and severity of exacerbations varies between patients.
BronchodilatorsBronchodilators increase FEV1, reduce dynamic hyperinflation, at rest and during exercise,104,105 and improve exercise performance. Bronchodilator medications are usually given on a regular basis to prevent or reduce symptoms. Toxicity is dose-related.
Beta2-agonists. Beta2-agonists, including short-acting (SABA) and long-acting (LABA) agents, relax airway smooth muscle. Stimulation of beta2-adrenergic receptors can produce resting sinus tachycardia and precipitate cardiac rhythm disturbances in susceptible patients. Exaggerated somatic tremor occurs in some patients treated with higher doses of beta2-agonists.
Antimuscarinic drugs. Ipratropium, a short acting muscarinic antagonist, provides small benefits over short-acting beta2-agonist in terms of lung function, health status and requirement for oral steroids.106 Long acting muscarinic antagonist (LAMA) treatment improves symptoms and health status,107,108 improves the effectiveness of pulmonary rehabilitation109,110 and reduces exacerbations and related hospitalizations.107 Clinical trials have shown a greater effect on exacerbation rates for LAMA treatment (tiotropium) versus LABA treatment.111,112 An unexpected small increase in cardiovascular events was reported in COPD patients regularly treated with ipratropium bromide.113,114 A large trial reported no difference in mortality, cardiovascular morbidity or exacerbation rates when using tiotropium as a dry-powder inhaler compared to a mist delivered by the Respimat® inhaler.115
Methylxanthines. Theophylline exerts a modest bronchodilator effect in stable COPD,116 and improves FEV1 and breathlessness when added to salmeterol.117,118 There is limited and contradictory evidence regarding the effect of low-dose theophylline on exacerbation rates.119,120 Toxicity is dose-related, which is a problem as most of the benefit occurs when near-toxic doses are given.116,121
Combination bronchodilator therapyCombining bronchodilators with different mechanisms and durations of action may increase the degree of bronchodilation with a lower risk of side-effects compared to increasing the dose of a single bronchodilator (Table 3).122 There are numerous combinations of a LABA and LAMA in a single inhaler available (Table S3). These combinations improve lung function compared to placebo122 and have a greater impact on patient reported outcomes compared to monotherapies.123–126 LABA/LAMA improves symptoms and health status in COPD patients,127 is more effective than long-acting bronchodilator monotherapy for preventing exacerbations,128 and decreases exacerbations to a greater extent than ICS/LABA combination.129
Bronchodilators in stable COPD.
| • Inhaled bronchodilators in COPD are central to symptom management and commonly given on a regular basis to prevent or reduce symptoms (Evidence A). |
| • Regular and as-needed use of SABA or SAMA improves FEV1 and symptoms (Evidence A). |
| • Combinations of SABA and SAMA are superior compared to either medication alone in improving FEV1 and symptoms (Evidence A). |
| • LABAs and LAMAs significantly improve lung function, dyspnea, health status, and reduce exacerbation rates (Evidence A). |
| • LAMAs have a greater effect on exacerbation reduction compared with LABAs (Evidence A) and decrease hospitalizations (Evidence B). |
| • Combination treatment with a LABA and LAMA increases FEV1 and reduces symptoms compared to monotherapy (Evidence A). |
| • Combination treatment with a LABA and LAMA reduces exacerbations compared to monotherapy (Evidence B) or ICS/LABA (Evidence B). |
| • Tiotropium improves the effectiveness of pulmonary rehabilitation in increasing exercise performance (Evidence B). |
| • Theophylline exerts a small bronchodilator effect in stable COPD (Evidence A) that is associated with modest symptomatic benefits (Evidence B). |
Exacerbations represent the main clinically relevant end-point used for the efficacy assessment of anti-inflammatory drugs (Table 4).
Anti-inflammatory therapy in stable COPD.
| Inhaled corticosteroids |
| • An ICS combined with a LABA is more effective than the individual components in improving lung function and health status and reducing exacerbations in patients with exacerbations and moderate to very severe COPD (Evidence A). |
| • Regular treatment with ICS increases the risk of pneumonia especially in those with severe disease (Evidence A). |
| • Triple inhaled therapy of ICS/LAMA/LABA improves lung function, symptoms and health status (Evidence A) and reduces exacerbations (Evidence B) compared to ICS/LABA or LAMA monotherapy. |
| Oral glucocorticoids |
| • Long-term use of oral glucocorticoids has numerous side effects (Evidence A) with no evidence of benefits (Evidence C). |
| PDE4 inhibitors |
| • In patients with chronic bronchitis, severe to very severe COPD and a history of exacerbations: |
| ○ A PDE4 inhibitor improves lung function and reduces moderate and severe exacerbations (Evidence A). |
| ○ A PDE4 inhibitor improves lung function and decreases exacerbations in patients who are on fixed-dose LABA/ICS combinations (Evidence B). |
| Antibiotics |
| • Long-term azithromycin and erythromycin therapy reduces exacerbations over one year (Evidence A). |
| Treatment with azithromycin is associated with an increased incidence of bacterial resistance (Evidence A) and hearing test impairment (Evidence B). |
| Mucolytics/antioxidants |
| • Regular use of NAC and carbocysteine reduces the risk of exacerbations in select populations (Evidence B). |
| Other anti-inflammatory agents |
| • Simvastatin does not prevent exacerbations in COPD patients at increased risk of exacerbations and without indications for statin therapy (Evidence A). However, observational studies suggest that statins may have positive effects on some outcomes in patients with COPD who receive them for cardiovascular and metabolic indications (Evidence C). |
| • Leukotriene modifiers have not been tested adequately in COPD patients. |
In patients with moderate to very severe COPD and exacerbations, an inhaled corticosteroid (ICS) combined with a LABA is more effective than either component alone in improving lung function, health status and reducing exacerbations.130,131 However, survival is not affected by combination therapy.132,133
ICS use has a higher prevalence of oral candidiasis, hoarse voice, skin bruising and pneumonia.134 Patients at higher risk of pneumonia include those who currently smoke, are aged>55 years, have a history of prior exacerbations or pneumonia, a body mass index (BMI)<25kg/m2, a poor MRC dyspnea grade and/or severe airflow limitation.135
Results from RCTs have yielded variable results regarding the risk of decreased bone density and fractures with ICS treatment.101,136–139 Observational studies suggest that ICS treatment could be associated with increased risks of diabetes/poor control of diabetes,140 cataracts,141 and mycobacterial infection142 including tuberculosis.143,144
ICS withdrawal. Withdrawal studies provide equivocal results regarding the consequences of withdrawal on lung function, symptoms and exacerbations.145–149
Triple inhaled therapyCombination of LABA plus LAMA plus ICS (triple therapy) may improve lung function and patient reported outcomes.150–153 and reduce exacerbation risk.151,154–156 However, one RCT failed to demonstrate any benefit of adding an ICS to LABA plus LAMA on exacerbations.157 More evidence is needed to compare the benefits of triple therapy (LABA/LAMA/ICS) to LABA/LAMA.
Oral glucocorticoidsOral glucocorticoids have no role in the chronic daily treatment in COPD because of a lack of benefit balanced against a high rate of systemic complications.
Phosphodiesterase-4 inhibitorsRoflumilast reduces moderate and severe exacerbations treated with systemic corticosteroids in patients with chronic bronchitis, severe to very severe COPD, and a history of exacerbations.158 Phosphodiesterase-4 (PDE4) inhibitors have more adverse effects than inhaled medications for COPD.159 The most frequent are diarrhea, nausea, reduced appetite, weight loss, abdominal pain, sleep disturbance, and headache. Roflumilast should be avoided in underweight patients and used with caution in patients with depression.
AntibioticsAzithromycin (250mg/day or 500mg three times per week) or erythromycin (500mg two times per day) for one year reduces the risk of exacerbations in patients prone to exacerbations.160–162 Azithromycin use showed a reduced exacerbation rate in former smokers only and was associated with an increased incidence of bacterial resistance and impaired hearing tests.162 Pulse moxifloxacin therapy in patients with chronic bronchitis and frequent exacerbations does not reduce exacerbation rate.163
Mucolytic (mucokinetics, mucoregulators) and antioxidant agents (N-acetylcysteine, carbocysteine)Regular treatment with mucolytics such as carbocysteine and N-acetylcysteine may reduce exacerbations and modestly improve health status in patients not receiving ICS.164,165
Other drugs with anti-inflammatory potentialAlthough RCTs suggest that immunoregulators decrease the severity and frequency of exacerbations,166,167 the long-term effects of this therapy are unknown. Nedocromil and leukotriene modifiers have not been adequately tested in COPD.168 There was no evidence of benefit, and some evidence of harm, following treatment with an anti-TNF-alpha antibody (infliximab) in moderate to severe COPD.169 Simvastatin did not prevent exacerbations in patients with COPD who had no metabolic or cardiovascular indication for statin treatment.170 An association between statin use and improved outcomes has been reported in observational studies of patients with COPD who received them for cardiovascular and metabolic indications.171 There is no evidence that vitamin D supplementation reduces exacerbations in unselected patients.172
Issues related to inhaled deliveryObservational studies have identified a significant relationship between poor inhaler use and symptom control in COPD.173 Determinants of poor inhaler technique include older age, use of multiple devices, and lack of previous education on inhaler technique.174 Education improves inhalation technique in some but not all patients,174 especially when the “teach-back” approach is implemented.175
Other pharmacologic treatments for COPD are summarized in Table S4 in the Supplementary Appendix.
Alpha-1 antitrypsin augmentation therapy. Observational studies suggest a reduction in spirometric progression in alpha-1 antitrypsin deficiency patients treated with augmentation therapy versus non-treated patients.176 Studies using sensitive parameters of emphysema progression determined by CT scans provide evidence for an effect on preserving lung tissue compared to placebo.177–179
Antitussives. The role of antitussives in patients with COPD is inconclusive.180
Vasodilators. Available studies report worsening gas exchange181 with little improvement in exercise capacity or health status in COPD patients.182,183
Rehabilitation, Education, and Self-ManagementPulmonary RehabilitationPulmonary rehabilitation is a comprehensive intervention based on thorough patient assessment followed by patient-tailored therapies (e.g., exercise training, education, self-management interventions aimed at behavior changes to improve physical and psychological condition and promote adherence to health-enhancing behaviors in patients with COPD).184 The benefits of pulmonary rehabilitation are considerable (Table S5 in the Supplementary Appendix). Pulmonary rehabilitation can reduce readmissions and mortality in patients following a recent exacerbation (≤ 4 weeks from prior hospitalization).185 Initiating pulmonary rehabilitation before hospital discharge, however, may compromise survival.186
Pulmonary rehabilitation represents integrated patient management that includes a range of healthcare professionals187 and sites, including hospital inpatient and outpatient settings and/or the patient's home.184
Education, Self-Management, and Integrative CareEducation. Smoking cessation, correct use of inhaler devices, early recognition of exacerbation, decision making, when to seek help, surgical interventions, and the consideration of advance directives, are examples of educational topics.
Self-management. Self-management interventions that use written negotiated action plans for worsening symptoms may lead to less respiratory-related hospitalization and all cause hospitalizations and improved health status.188 The health benefits of COPD self-management programs may be negated by increased mortality.189,190 Generalization to real life remains difficult.
Integrated care programs. Integrated care programs improve several clinical outcomes, although not mortality.191 However, a large multi-center study within an existing well-organized system of care did not confirm this.192 Delivering integrated interventions by telemedicine provided no significant benefit.193
Supportive, Palliative, End-of-Life, and Hospice CareSymptom Control and Palliative CareThe goal of palliative care is to prevent and relieve suffering, and to improve quality of life for patients and their families, regardless of the stage of disease or the need for other therapies.194 Palliation efforts should be focused on the relief of dyspnea, pain, anxiety, depression, fatigue, and poor nutrition.
End-of-Life and Hospice CareEnd of life care discussions should include patients and their families.195 Advance care planning can reduce anxiety for patients and their families, ensure that care is consistent with their wishes and avoid unnecessary, unwanted and costly invasive therapies196,197 Table S6 in the Supplementary Appendix summarizes the approach to palliation, end-of-life and hospice care
Other TreatmentsOxygen Therapy and Ventilatory SupportOxygen therapy. The long-term administration of oxygen (> 15hours per day) to patients with chronic respiratory failure increases survival in patients with severe resting hypoxemia.198 Long term oxygen therapy does not lengthen time to death or first hospitalization or provide sustained benefit for any of the measured outcomes in patients with stable COPD and resting or exercise-induced moderate arterial oxygen desaturation.199
Ventilatory support. Whether to use NPPV chronically at home to treat patients with acute on chronic respiratory failure following hospitalization remains undetermined. Retrospective studies have provided inconclusive data.200,201 RCTs have yielded conflicting data on the use of home NPPV on survival and re-hospitalization in chronic hypercapnic COPD.202–205 In patients with both COPD and obstructive sleep apnea continuous positive airway pressure improves survival and avoids hospitalization (Table S7 in the Supplementary Appendix).206
Interventional TherapySurgical InterventionsLung volume reduction surgery. A RCT confirmed that COPD patients with upper-lobe emphysema and low post-rehabilitation exercise capacity experienced improved survival when treated with lung volume reduction surgery (LVRS) compared to medical treatment.207 In patients with high post-pulmonary rehabilitation exercise capacity, no difference in survival was noted after LVRS, although health status and exercise capacity improved. LVRS has been demonstrated to result in higher mortality than medical management in severe emphysema patients with an FEV1 ≤ 20% predicted and either homogeneous emphysema in high resolution computed tomography or a DLCO of ≤ 20% of predicted.208
Bullectomy. In selected patients with relatively preserved underlying lung, bullectomy is associated with decreased dyspnea, improved lung function and exercise tolerance.209
Lung transplantation. In selected patients lung transplantation has been shown to improve health status and functional capacity but not to prolong survival.209–211 Bilateral lung transplantation has been reported to have longer survival than single lung transplantation in COPD patients, especially those<60 years of age.212
Bronchoscopic Interventions to Reduce Hyperinflation in Severe EmphysemaLess invasive bronchoscopic approaches to lung reduction have been developed.213 Prospective studies have shown that the use of bronchial stents is not effective214 while use of lung sealant caused significant morbidity and mortality.215 A RCT of endobronchial valve placement showed statistically significant improvements in FEV1 and 6-minute walk distance compared to control therapy at 6 months post intervention216 but the magnitude of the observed improvements was not clinically meaningful. Subsequently, efficacy of the same endobronchial valve has been studied in patients with heterogeneous,217 or heterogeneous and homogenous emphysema218 with mixed outcomes.
Two multicenter trials have examined nitinol coils implanted into the lung compared to usual care reported increases in 6minute walk distance with coil treatment compared to control and smaller improvements in FEV1 and quality of life measured by St George's Respiratory Questionnaire.219,220
Additional data are needed to define the optimal patient population to receive a specific bronchoscopic lung volume technique and to compare the long-term durability of improvements in functional or physiological performance to LVRS relative to side effects.220
Key points for interventional therapy in stable COPD are summarized in Table S8 in the Supplementary Appendix.
Management of Stable COPDKey Points
- •
The management strategy for stable COPD should be based on individualized symptom assessment and future risk of exacerbations.
- •
All individuals who smoke should be supported to quit.
- •
The main treatment goals are reduction of symptoms and future risk of exacerbations.
- •
Management strategies are not limited to pharmacologic treatments, and should be complemented by appropriate non-pharmacologic interventions.
Effective COPD management should be based on an individualized assessment to reduce both current symptoms and future risks of exacerbations (Figure S1 in the Supplementary Appendix).
We propose personalization of initiating and escalating/de-escalating treatments based on the level of symptoms and an individual's risk of exacerbations. The basis for these recommendations is partially based on evidence generated in RCTs. These recommendations are intended to support clinician decision-making.
Identify and Reduce Exposure to Risk FactorsCigarette smoking is the most commonly encountered and easily identifiable risk factor for COPD; smoking cessation should be continually encouraged for current smokers. Reduction of total personal exposure to occupational dusts, fumes, and gases, and to indoor and outdoor air pollutants, should be addressed.
Treatment of Stable COPDPharmacologic TreatmentPharmacologic therapies can reduce symptoms, the risk and severity of exacerbations, and improve health status and exercise tolerance. The choice within each class depends on the availability of medication and the patient's response and preference (Tables 5–7).
Key points for the use of bronchodilators.
| • LABAs and LAMAs are preferred over short-acting agents except for patients with only occasional dyspnea (Evidence A). |
| • Patients may be started on single long-acting bronchodilator therapy or dual long-acting bronchodilator therapy. In patients with persistent dyspnea on one bronchodilator treatment should be escalated to two (Evidence A). |
| • Inhaled bronchodilators are recommended over oral bronchodilators (Evidence A). |
| • Theophylline is not recommended unless other long-term treatment bronchodilators are unavailable or unaffordable (Evidence B). |
Key points for the use of anti-inflammatory agents.
| • Long-term monotherapy with ICS is not recommended (Evidence A). |
| • Long-term treatment with ICS may be considered in association with LABAs for patients with a history of exacerbations despite appropriate treatment with long-acting bronchodilators (Evidence A). |
| • Long-term therapy with oral corticosteroids is not recommended (Evidence A). |
| • In patients with exacerbations despite LABA/ICS or LABA/LAMA/ICS, chronic bronchitis and severe to very severe airflow obstruction, the addition of a PDE4 inhibitor can be considered (Evidence B). |
| • In former smokers with exacerbations despite appropriate therapy, macrolides can be considered (Evidence B). |
| Statin therapy is not recommended for prevention of exacerbations (Evidence A). |
| Antioxidant mucolytics are recommended only in selected patients (Evidence A). |
Key points for the use of other pharmacologic treatments.
| • Patients with severe hereditary alpha-1 antitrypsin deficiency and established emphysema may be candidates for alpha-1 antitrypsin augmentation therapy (Evidence B). |
| • Antitussives cannot be recommended (Evidence C). |
| • Drugs approved for primary pulmonary hypertension are not recommended for patients with pulmonary hypertension secondary to COPD (Evidence B). |
| • Low-dose long acting oral and parenteral opioids may be considered for treating dyspnea in COPD patients with severe disease (Evidence B). |
A proposed model for the initiation, and then subsequent escalation and/or de-escalation of pharmacologic management according to the individualized assessment of symptoms and exacerbation risk is shown in Fig. 3. In past GOLD Reports, recommendations were only given for initial therapy. However, many COPD patients are already on treatment and return with persistent symptoms after initial therapy, or less commonly with resolution of some symptoms that may subsequently require less therapy. Therefore, we now suggest escalation and de-escalation strategies. The recommendations are based on available efficacy and safety data. We acknowledge that treatment escalation has not been systematically tested; trials of de-escalation are also limited and only include ICS. There is a lack of direct evidence supporting the therapeutic recommendations for patients in groups C and D. These recommendations will be re-evaluated as additional data become available.
All Group A patients should be offered a bronchodilator to reduce breathlessness. This can be either a short or a long-acting bronchodilator based on the individual patient's preference. The bronchodilator should be continued if symptomatic benefit is noted.
Group BInitial therapy should be a long acting bronchodilator. Long-acting bronchodilators are superior to short-acting bronchodilators taken intermittently.106,221 There is no evidence to recommend one class of long-acting bronchodilators over another for symptom relief, the choice should depend on individual patient response.
For patients with persistent breathlessness on monotherapy222 the use of two bronchodilators is recommended. For patients with severe breathlessness, initial therapy with two bronchodilators may be considered.
Group CInitial therapy should be a single long acting bronchodilator. In two head-to head comparisons112,223 the LAMA tested superior to the LABA regarding exacerbation prevention, therefore we recommend initiating a LAMA in this group.
Patients with persistent exacerbations may benefit from adding a second long acting bronchodilator (LABA/LAMA), or using a combination of a long acting beta2-agonist and an inhaled corticosteroid (LABA/ICS). As ICS increases the risk for developing pneumonia, our primary choice is LABA/LAMA.
Group DWe recommend initiating a LABA/LAMA combination because:
- •
In studies with patient reported outcomes as the primary endpoint, LABA/LAMA combinations showed superior results compared to a single bronchodilator.
- •
LABA/LAMA combination was superior to LABA/ICS combination in preventing exacerbations and improving other patient reported outcomes in Group D patients.
- •
Group D patients are at higher risk for pneumonia when receiving ICS treatment.111,135
If a single bronchodilator is initially chosen, a LAMA is preferred for exacerbation prevention based on comparison to LABAs.
LABA/ICS may be the first choice for initial therapy in some patients. These patients may have a history and/or findings suggestive of asthma-COPD overlap and/or high blood eosinophil counts.
In patients who develop additional exacerbations on LABA/LAMA therapy we suggest two alternative pathways:
- ∘
Escalation to LABA/LAMA/ICS.
- ∘
Switch to LABA/ICS. If LABA/ICS therapy does not positively impact exacerbations/symptoms, a LAMA can be added.
If patients treated with LABA/LAMA/ICS still have exacerbations the following options may be considered:
- ∘
Add roflumilast. This may be considered in patients with an FEV1<50% predicted and chronic bronchitis,224 particularly if they experienced at least one hospitalization for an exacerbation in the previous year.225
- ∘
Add a macrolide in former smokers. The possibility of developing resistant organisms should be factored into the decision making.
- ∘
Stopping ICS. This recommendation is supported by data that shows an elevated risk of adverse effects (including pneumonia) and no significant harm from ICS withdrawal.
An individual patient's evaluation and risk assessment (e.g., exacerbations, patient's needs, preferences, and personal goals) should aid the design of personalized self-management.
Pulmonary rehabilitation programsPatients with high symptom burden and risk of exacerbations (Groups B, C and D), should take part in a full rehabilitation program that considers the individual's characteristics and comorbidities.184,226,227
Exercise trainingA combination of constant load or interval training with strength training provides better outcomes than either method alone.228 Adding strength training to aerobic training is effective in improving strength, but does not improve health status or exercise tolerance.229 Upper extremity exercise training improves arm strength and endurance and improves capacity for upper extremity activities.230
Self-management educationAn educational program should include smoking cessation; basic information about COPD; aspects of medical treatment (respiratory medications and inhalation devices); strategies to minimize dyspnea; advice about when to seek help; and possibly a discussion of advance directives and end-of-life issues.
End-of-life and palliative carePatients should be informed that should they become critically ill, they or their family members may need to decide whether a course of intensive care is likely to achieve their personal goals of care. Simple, structured conversations about these possible scenarios should be discussed while patients are in their stable state.231
Nutritional supportFor malnourished patients with COPD nutritional supplementation is recommended.
VaccinationInfluenza vaccination is recommended for all patients with COPD. Pneumococcal vaccinations, PCV13 and PPSV23, are recommended for all patients>65 years of age. The PPSV23 is also recommended for younger COPD patients with significant comorbid conditions including chronic heart or lung disease.232
Oxygen therapyLong-term oxygen therapy is indicated for stable patients who have:
- •
PaO2 at or below 7.3kPa (55mmHg) or SaO2 at or below 88%, with or without hypercapnia confirmed twice over a three-week period; or
- •
PaO2 between 7.3kPa (55mmHg) and 8.0kPa (60mmHg), or SaO2 of 88%, if there is evidence of pulmonary hypertension, peripheral edema suggesting congestive cardiac failure, or polycythemia (hematocrit>55%).
NIV is occasionally used in patients with stable very severe COPD. NIV may be considered in a selected group of patients, particularly those with pronounced daytime hypercapnia and recent hospitalization, although contradictory evidence exists regarding its effectiveness.233 In patients with both COPD and obstructive sleep apnea continuous positive airway pressure is indicated.206
Interventional bronchoscopy and surgery- •
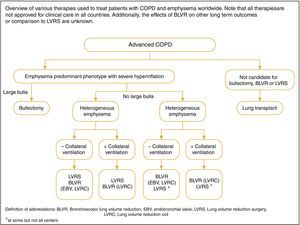
In selected patients with heterogeneous or homogenous emphysema and significant hyperinflation refractory to optimized medical care, surgical or bronchoscopic modes of lung volume reduction (e.g., endobronchial one-way valves or lung coils) may be considered.234
- •
In selected patients with a large bulla, surgical bullectomy may be considered.
- •
In selected patients with very severe COPD and without relevant contraindications, lung transplantation may be considered.
Choosing bronchoscopic lung reduction or LVRS to treat hyperinflation in an emphysematous patient depends on a number of factors that include: the extent and pattern of emphysema identified on HRCT; the presence of interlobar collateral ventilation measured by fissure integrity on HRCT or physiological assessment (endoscopic balloon occlusion and flow assessment); local proficiency in the performance of the procedures; and patient and provider preferences. An algorithm depicting the various interventions based on radiological and physiological features is shown in Fig. 4.
Criteria for referral for lung transplantation include COPD with progressive disease, not a candidate for endoscopic or surgical lung volume reduction, BODE index of 5 to 6, Pco2>50mmHg or 6.6kPa and/or Pao2<60mmHg or 8kPa, and FEV1<25% predicted.235 Recommended criteria for listing include one of the following: BODE index>7, FEV1<15-20% predicted, three or more severe exacerbations during the preceding year, one severe exacerbation with acute hypercapnic respiratory failure, or moderate to severe pulmonary hypertension.235,236
Key points for the use of non-pharmacologic treatments are summarized in Table S9 in the Supplementary Appendix.
Monitoring and Follow-UpRoutine follow-up of COPD patients is essential. Symptoms, exacerbations and objective measures of airflow limitation should be monitored to determine when to modify management and to identify any complications and/or comorbidities that may develop. In order to adjust therapy appropriately as the disease progresses, each follow-up visit should include a discussion of the current therapeutic regimen. Symptoms that indicate worsening or development of another comorbid condition should be evaluated and treated.
Management of ExacerbationsKey Points
- •
An exacerbation of COPD is an acute worsening of respiratory symptoms that results in additional therapy.
- •
Exacerbations can be precipitated by several factors. The most common causes are respiratory tract infections.
- •
The goal for treatment of exacerbations is to minimize the negative impact of the current exacerbation and to prevent subsequent events.
- •
Short-acting inhaled beta2-agonists, with or without short-acting anticholinergics, are recommended as the initial bronchodilators to treat an acute exacerbation.
- •
Maintenance therapy with long-acting bronchodilators should be initiated as soon as possible before hospital discharge.
- •
Systemic corticosteroids improve lung function (FEV1), oxygenation and shorten recovery time and hospitalization duration.
- •
Antibiotics, when indicated, shorten recovery time, reduce the risk of early relapse, treatment failure, and hospitalization duration.
- •
Methylxanthines are not recommended due to side effects.
- •
Non-invasive mechanical ventilation should be the first mode of ventilation used to treat acute respiratory failure.
- •
Following an exacerbation, appropriate measures for exacerbation prevention should be initiated.
Exacerbations are important events in the management of COPD because they negatively impact health status, rates of hospitalization and readmission, and disease progression.237,238 COPD exacerbations are complex events usually associated with increased airway inflammation, increased mucus production and marked gas trapping. Increased dyspnea is the key symptom of an exacerbation. Other symptoms include increased sputum purulence and volume, together with increased cough and wheeze.239 As comorbidities are common in COPD patients, exacerbations must be differentiated from acute coronary syndrome, worsening congestive heart failure, pulmonary embolism and pneumonia.
COPD exacerbations are classified as:
- •
Mild (treated with short acting bronchodilators only, SABDs)
- •
Moderate (treated with SABDs plus antibiotics and/or oral corticosteroids) or
- •
Severe (patient requires hospitalization or visits the emergency room). Severe exacerbations may be associated with acute respiratory failure.
Exacerbations are mainly triggered by respiratory viral infections although bacterial infections and environmental factors may also initiate and/or amplify these events.240
Exacerbations can be associated with increased sputum production and, if purulent, increased bacteria may be found in the sputum239,241,242 Some evidence supports the concept that eosinophils are increased in the airways, lung, and blood in a significant proportion of patients with COPD. Exacerbations associated with an increase in sputum or blood eosinophils may be more responsive to systemic steroids243 although more prospective data are needed.243
Symptoms usually last between 7 to 10 days during an exacerbation, but some events may last longer. At 8 weeks, 20% of patients have not recovered to their pre-exacerbation state.244 COPD exacerbations increase susceptibility to additional events.59,245
COPD patients susceptible to frequent exacerbations (defined as ≥ 2 exacerbations per year) have worse health status and morbidity than patients with less frequent exacerbations.238 Other factors associated with an increased risk of acute exacerbations and/or severity of exacerbations include an increase in the ratio of the pulmonary artery to aorta cross sectional dimension (i.e., ratio>1),246 a greater percentage of emphysema or airway wall thickness247 measured by chest CT imaging and the presence of chronic bronchitis.248,249
Treatment OptionsTreatment SettingThe goals of exacerbation treatment are to minimize the negative impact of the current exacerbation, and to prevent the development of subsequent events.250 Depending on the severity of an exacerbation and/or the severity of the underlying disease, an exacerbation can be managed in either the outpatient or inpatient setting. More than 80% of exacerbations are managed on an outpatient basis with bronchodilators, corticosteroids, and antibiotics.251–253
The indications for hospitalization during a COPD exacerbation are shown in Table S10 in the Supplementary Appendix. When patients with a COPD exacerbation come to the emergency department, they should be given supplemental oxygen and assessed to determine whether the exacerbation is life-threatening and requires consideration for non-invasive ventilation and ICU or respiratory unit hospitalization.
Long-term prognosis following hospitalization for COPD exacerbation is poor; five-year mortality rate is about 50%.254 Factors associated with poor outcome include older age, lower body mass index, comorbidities (e.g., cardiovascular disease or lung cancer), previous hospitalizations for COPD exacerbations, clinical severity of the index exacerbation, and need for long-term oxygen therapy at discharge.255,256 Patients with a higher prevalence and severity of respiratory symptoms, poorer quality of life, worse lung function, lower exercise capacity, lower lung density and thickened bronchial walls on CT-scan are at increased mortality risk following an acute exacerbation.257
Key points for the management of all exacerbations are given in Table 8.
Key points for the management of exacerbations.
| • Short-acting inhaled beta2-agonists, with or without short-acting anticholinergics, are recommended as the initial bronchodilators to treat an acute exacerbation (Evidence C). |
| • Systemic corticosteroids improve lung function (FEV1), oxygenation and shorten recovery time and hospitalization duration. Duration of therapy should not be more than 5-7 days (Evidence A). |
| • Antibiotics, when indicated, can shorten recovery time, reduce the risk of early relapse, treatment failure, and hospitalization duration. Duration of therapy should be 5-7 days (Evidence B). |
| • Methylxanthines are not recommended due to increased side effect profiles (Evidence B). |
| • NIV should be the first mode of ventilation used in COPD patients with acute respiratory failure who have no absolute contraindication because it improves gas exchange, reduces work of breathing and the need for intubation, decreases hospitalization duration and improves survival (Evidence A). |
The most commonly used classes of medications for COPD exacerbations are bronchodilators, corticosteroids, and antibiotics.
Bronchodilators. Short-acting inhaled beta2-agonists, with or without short-acting anticholinergics, are the initial bronchodilators recommended for acute treatment of exacerbations.258,259 There are no significant differences in FEV1 when using metered dose inhalers (MDI) (with or without a spacer device) or nebulizers to deliver the agent,260 although the latter may be an easier delivery method for sicker patients. Intravenous methylxanthines are not recommended due to side effects.261,262
Glucocorticoids. Systemic glucocorticoids in COPD exacerbations shorten recovery time and improve FEV1. They also improve oxygenation,263–266 the risk of early relapse, treatment failure,267 and the length of hospitalization.263,265,268 A dose of 40mg prednisone per day for 5 days is recommended.269 Therapy with oral prednisolone is equally effective to intravenous administration.270 Glucocorticoids may be less efficacious to treat exacerbations in patients with lower blood eosinophil levels.59,243,271
Antibiotics. The use of antibiotics in exacerbations remains controversial.272–274 Evidence supports the use of antibiotics in patients with exacerbations and increased sputum purulence.273,274 One review reported that antibiotics reduce the risk of short-term mortality by 77%, treatment failure by 53% and sputum purulence by 44%275 Procalcitonin-guided antibiotic treatment may reduce antibiotic exposure and side effects with the same clinical efficacy.276,277 A study in patients with exacerbations requiring mechanical ventilation (invasive or noninvasive) reported increased mortality and a higher incidence of secondary nosocomial pneumonia when antibitoics were not given.278 Antibiotics should be given to patients with acute exacerbations who have three cardinal symptoms: increase in dyspnea, sputum volume, and sputum purulence; have two of the cardinal symptoms, if increased purulence of sputum is one of the two symptoms; or require mechanical ventilation (invasive or noninvasive).239,240 The recommended length of antibiotic therapy is 5-7 days.279
Antibiotic choice should be based on the local bacterial resistance pattern. Usual initial empirical treatment is an aminopenicillin with clavulanic acid, a macrolide, or a tetracycline. In patients with frequent exacerbations, severe airflow limitation,280,281 and/or exacerbations requiring mechanical ventilation,282 cultures from sputum or other materials from the lung should be performed to identify the presence of resistant pathogens. Administration route depends on the patient's ability to eat and the pharmacokinetics of the antibiotic.
Respiratory SupportOxygen therapy. Supplemental oxygen should be titrated to improve hypoxemia with a target saturation of 88-92%.283 Once oxygen is started, blood gases should be checked to ensure satisfactory oxygenation without carbon dioxide retention and/or worsening acidosis.
Ventilatory support. Some patients require admission to the intensive care unit. Admission of patients with severe exacerbations to intermediate or special respiratory care units may be appropriate if adequate personnel skills and equipment exist to manage acute respiratory failure.
Noninvasive mechanical ventilation. NIV is preferred over invasive ventilation as the initial mode of ventilation to treat acute respiratory failure in patients hospitalized for acute exacerbations of COPD. NIV has been studied in RCTs showing a success rate of 80-85%.284–288 Mortality and intubation rates are reduced by NIV.284,289–291
Invasive mechanical ventilation. The indication for initiating invasive mechanical ventilation during an exacerbation includes failure of an initial trial of NIV.292 In patients who fail non-invasive ventilation as initial therapy and receive invasive ventilation as subsequent rescue therapy, morbidity, hospital length of stay and mortality are greater.287
Hospital Discharge and Follow-UpLack of spirometric assessment and arterial blood gas analysis have been associated with re-hospitalization and mortality.293 Mortality relates to patient age, the presence of acidotic respiratory failure, the need for ventilatory support and comorbidities including anxiety and depression.294
The introduction of care bundles at hospital discharge to include education, optimization of medication, supervision and correction of inhaler technique, assessment and optimal management of comorbidities, early rehabilitation, telemonitoring and continued patient contact have been investigated.295 There is insufficient data that they influence readmission rates, short-term mortality293,294,296,297 or cost-effectiveness.294
Early follow-up (< 30 days) following discharge should be undertaken when possible and has been related to less exacerbation-related readmissions.186,298 Early follow-up permits a careful review of discharge therapy and an opportunity to make changes in therapy. Patients not attending early follow-up have increased 90-day mortality.
Additional follow-up at three months is recommended to ensure return to a stable state and review of patient's symptoms, lung function (by spirometry), and when possible the assessment of prognosis using multiple scoring systems such as BODE.298,299 An assessment of the presence and management of comorbidities should also be undertaken (Table S11 in the Supplementary Appendix).300
Prevention of ExacerbationsAfter an acute exacerbation, measures for prevention of further exacerbations should be initiated (Table S12 in the Supplementary Appendix).
COPD and ComorbiditiesKey Points
- •
COPD often coexists with other diseases (comorbidities) that may significantly impact patient outcome.
- •
The presence of comorbidities should not alter COPD treatment and comorbidities should be treated per usual standards regardless of the presence of COPD.
- •
When COPD is part of a multi-morbidity care plan, attention should be directed to ensure simplicity of treatment and minimize polypharmacy.
COPD often coexists with other diseases (comorbidities) that may have a significant impact on prognosis.63,301–307 Some of these arise independently of COPD whereas others may be causally related, either with shared risk factors, or by one disease increasing the risk or compounding the severity of the other.308 Management of the COPD patient must include identification and treatment of its comorbidities; the most common in COPD are outlined below.
Cardiovascular diseaseHeart failureThe prevalence of systolic or diastolic heart failure in COPD patients ranges from 20 to 70%.309 Unrecognized heart failure may mimic or accompany acute exacerbations of COPD; 40% of COPD patients that are mechanically ventilated because of hypercapnic respiratory failure have evidence of left ventricular dysfunction.310,311 Treatment with ß1-blockers improves survival in chronic heart failure and is recommended. Selective ß1-blockers should be used.312
Ischemic heart diseaseThere is an increased risk of myocardial damage in patients with concomitant ischemic heart disease who have an acute exacerbation of COPD. Patients who demonstrate abnormal cardiac troponins are at an increased risk of adverse outcomes including short-term (30 day) and long-term mortality.313
ArrhythmiasCardiac arrhythmias are common in COPD and vice versa. Atrial fibrillation is frequent and directly associated with FEV1. Bronchodilators have been previously described as potentially pro-arrhythmic agents314,315; however, evidence suggests an overall acceptable safety profile for long-acting beta2-agonists,316 anticholinergic drugs (and inhaled corticosteroids).103,115,253,317–322
Peripheral vascular diseaseIn a large cohort of patients with COPD of all degrees of severity, 8.8% were diagnosed with peripheral artery disease (PAD) that was higher than the prevalence in non-COPD controls (1.8%).323 COPD patients with PAD reported a worse functional capacity and worse health status compared to those without PAD.
HypertensionHypertension is likely to be the most frequently occurring comorbidity in COPD and may have implications for prognosis.308,324
OsteoporosisOsteoporosis is often associated with emphysema,325,326 decreased body mass index327 and low fat-free mass.328 Low bone mineral density and fractures are common in COPD patients even after adjustment for steroid use, age, pack-years of smoking, current smoking, and exacerbations.329,330 An association between inhaled corticosteroids and fractures has been found in pharmaco-epidemiological studies. Systemic corticosteroids significantly increase the risk of osteoporosis.
Anxiety and DepressionAnxiety and depression are both associated with a poor prognosis.331,332
COPD and Lung CancerThe association between emphysema and lung cancer is stronger than between airflow limitation and lung cancer.333–335 Increased age and greater smoking history further increases risk.336 Two studies of low-dose chest computed tomography (LDCT) screening report improved survival in subjects aged 55-74 years, current smokers or those who quit within the previous 15 years, with a smoking history of at least 30 pack-years.337,338 LDCT is now recommended in the U.S. for patients meeting these demographics; however, this is not a worldwide practice.
Metabolic Syndrome and DiabetesMetabolic syndrome and diabetes are more frequent in COPD and the latter is likely to affect prognosis.302 The prevalence of metabolic syndrome has been estimated to be more than 30%.339
Gastroesophageal RefluxGastroesophageal reflux is an independent risk factor for exacerbations and is associated with worse health status.251,340,341
BronchiectasisBronchiectasis is associated with longer exacerbations342 and increased mortality.300
Obstructive Sleep ApneaPatients with “overlap syndrome” (COPD and OSA) have a worse prognosis compared with COPD or OSA. Apneic events in patients with OSA and COPD have more profound hypoxemia and more cardiac arrhythmias343 and are more likely to develop daytime pulmonary hypertension344,345 than patients with just OSA or COPD alone.
Conflict of InterestsThe conflict of interest statement of the authors (GOLD-Disclosures-Authors) is available in the additional material of this article in its electronic version, at doi:10.1016/j.arbres.2017.02.001.
These authors contributed equally to the manuscript
Please cite this article as: Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Documento sobre la estrategia global para el diagnóstico, el manejo, y la prevención de la Enfermedad Pulmonar Obstructiva Crónica: Sumario ejecutivo de la GOLD. Arch Bronconeumol. 2017;53:128–149.














![Pharmacologic treatment algorithms by GOLD Grade [highlighted boxes and arrows indicate preferred treatment pathways]. Pharmacologic treatment algorithms by GOLD Grade [highlighted boxes and arrows indicate preferred treatment pathways].](https://static.elsevier.es/multimedia/15792129/0000005300000003/v1_201703240112/S1579212917300484/v1_201703240112/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w98FxLWLw1xoW2PaQDYY7RZU=)