Idiopathic pulmonary fibrosis (IPF) is a chronic, interstitial lung disease of unknown etiology.1
Its clinical course is highly unpredictable and is characterized by a progressive decline of lung function, usually measured by forced vital capacity (FVC).1
Nintedanib, a multitarget tyrosin kinase inhibitor, can slow the progression of IPF by reducing the rate of decline in FVC and the risk of exacerbations.2,3
Interestingly, a subgroup of patients treated with nintedanib shows a stability/improvement of FVC in the first year of treatment.4–6 No clinical, functional and radiological data at baseline were found predictive of the outcome in clinical trials.4 However, potentially predictive variables related to comorbidities, complications of disease (i.e., hospitalization and exacerbations), and treatment (i.e., dose reduction and drug discontinuation) have never been investigated.
The primary aim of the study was to evaluate the presence of predictors of stability/improvement of FVC (milliliters, ml) after one year of treatment in a cohort of patients with mild to moderate IPF treated with nintedanib.
This is a secondary analysis of an observational, retrospective, multicentre study aimed at assessing the decline of FVC after 12 months of nintedanib in patients aged>80 years, who were compared with younger patients.6 It was approved by the ethical committees of four participating hospitals. Written informed consent was provided by patients alive when the study was planned.
From October 2018 to February 2022 adult (i.e., ≥18 years old) patients with mild to moderate IPF treated with nintedanib, who completed at least three months of therapy were enrolled.6 Only patients who completed one year of treatment were considered eligible for the present analysis, regardless of age.
Exclusion criteria were the following: severe IPF (FVC<50% of the predicted value), refusal to sign the informed consent.
The following variables were studied as potential predictors: age, sex, Body Mass Index (BMI), Eastern Cooperative Oncology Group-Performance Status (ECOG-PS), smoking history, computed tomography (CT) pattern, presence of emphysema at CT scan, comorbidities, baseline FVC values, baseline diffusion of the lung for carbon monoxide (DLCO) values, Gender Age Physiology (GAP) and TORVAN index, hospitalization for respiratory cause, exacerbation, dose reduction and drug discontinuation.6
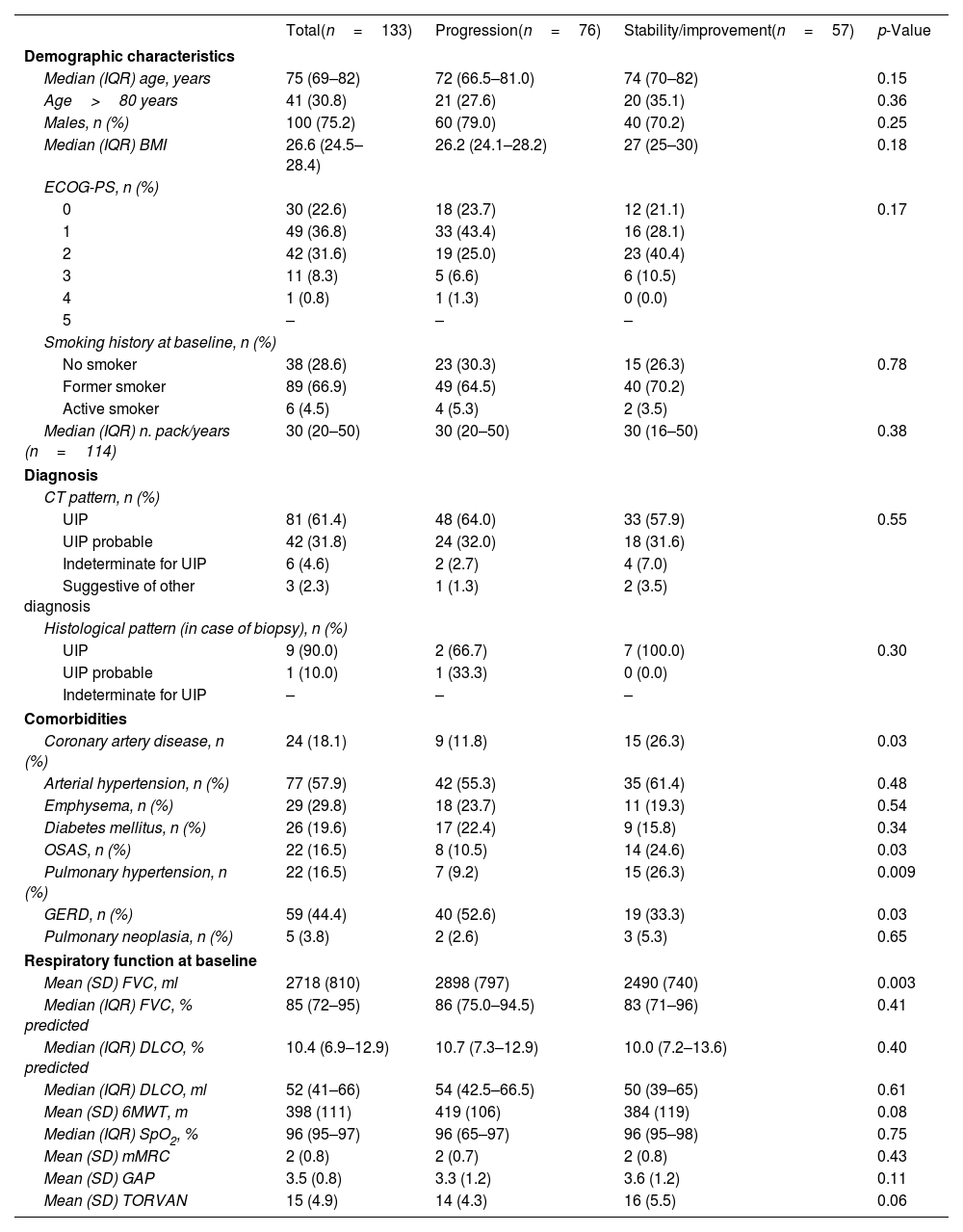
Demographic, clinical and functional characteristics of the whole cohort at baseline were previously described.6 Demographic, clinical and functional characteristics of the enrolled patients who completed one year of treatment are shown in Table 1.
Baseline characteristics of the patients who completed one year of treatment, stratified by progression or stability/improvement of FVC (ml).
| Total(n=133) | Progression(n=76) | Stability/improvement(n=57) | p-Value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Median (IQR) age, years | 75 (69–82) | 72 (66.5–81.0) | 74 (70–82) | 0.15 |
| Age>80 years | 41 (30.8) | 21 (27.6) | 20 (35.1) | 0.36 |
| Males, n (%) | 100 (75.2) | 60 (79.0) | 40 (70.2) | 0.25 |
| Median (IQR) BMI | 26.6 (24.5–28.4) | 26.2 (24.1–28.2) | 27 (25–30) | 0.18 |
| ECOG-PS, n (%) | ||||
| 0 | 30 (22.6) | 18 (23.7) | 12 (21.1) | 0.17 |
| 1 | 49 (36.8) | 33 (43.4) | 16 (28.1) | |
| 2 | 42 (31.6) | 19 (25.0) | 23 (40.4) | |
| 3 | 11 (8.3) | 5 (6.6) | 6 (10.5) | |
| 4 | 1 (0.8) | 1 (1.3) | 0 (0.0) | |
| 5 | – | – | – | |
| Smoking history at baseline, n (%) | ||||
| No smoker | 38 (28.6) | 23 (30.3) | 15 (26.3) | 0.78 |
| Former smoker | 89 (66.9) | 49 (64.5) | 40 (70.2) | |
| Active smoker | 6 (4.5) | 4 (5.3) | 2 (3.5) | |
| Median (IQR) n. pack/years (n=114) | 30 (20–50) | 30 (20–50) | 30 (16–50) | 0.38 |
| Diagnosis | ||||
| CT pattern, n (%) | ||||
| UIP | 81 (61.4) | 48 (64.0) | 33 (57.9) | 0.55 |
| UIP probable | 42 (31.8) | 24 (32.0) | 18 (31.6) | |
| Indeterminate for UIP | 6 (4.6) | 2 (2.7) | 4 (7.0) | |
| Suggestive of other diagnosis | 3 (2.3) | 1 (1.3) | 2 (3.5) | |
| Histological pattern (in case of biopsy), n (%) | ||||
| UIP | 9 (90.0) | 2 (66.7) | 7 (100.0) | 0.30 |
| UIP probable | 1 (10.0) | 1 (33.3) | 0 (0.0) | |
| Indeterminate for UIP | – | – | – | |
| Comorbidities | ||||
| Coronary artery disease, n (%) | 24 (18.1) | 9 (11.8) | 15 (26.3) | 0.03 |
| Arterial hypertension, n (%) | 77 (57.9) | 42 (55.3) | 35 (61.4) | 0.48 |
| Emphysema, n (%) | 29 (29.8) | 18 (23.7) | 11 (19.3) | 0.54 |
| Diabetes mellitus, n (%) | 26 (19.6) | 17 (22.4) | 9 (15.8) | 0.34 |
| OSAS, n (%) | 22 (16.5) | 8 (10.5) | 14 (24.6) | 0.03 |
| Pulmonary hypertension, n (%) | 22 (16.5) | 7 (9.2) | 15 (26.3) | 0.009 |
| GERD, n (%) | 59 (44.4) | 40 (52.6) | 19 (33.3) | 0.03 |
| Pulmonary neoplasia, n (%) | 5 (3.8) | 2 (2.6) | 3 (5.3) | 0.65 |
| Respiratory function at baseline | ||||
| Mean (SD) FVC, ml | 2718 (810) | 2898 (797) | 2490 (740) | 0.003 |
| Median (IQR) FVC, % predicted | 85 (72–95) | 86 (75.0–94.5) | 83 (71–96) | 0.41 |
| Median (IQR) DLCO, % predicted | 10.4 (6.9–12.9) | 10.7 (7.3–12.9) | 10.0 (7.2–13.6) | 0.40 |
| Median (IQR) DLCO, ml | 52 (41–66) | 54 (42.5–66.5) | 50 (39–65) | 0.61 |
| Mean (SD) 6MWT, m | 398 (111) | 419 (106) | 384 (119) | 0.08 |
| Median (IQR) SpO2, % | 96 (95–97) | 96 (65–97) | 96 (95–98) | 0.75 |
| Mean (SD) mMRC | 2 (0.8) | 2 (0.7) | 2 (0.8) | 0.43 |
| Mean (SD) GAP | 3.5 (0.8) | 3.3 (1.2) | 3.6 (1.2) | 0.11 |
| Mean (SD) TORVAN | 15 (4.9) | 14 (4.3) | 16 (5.5) | 0.06 |
BMI: body mass index; ECOG-PS: Eastern Cooperative Oncology Group- Performance Status; CT: computed tomography; UIP: usual interstitial pneumonia; OSAS: obstructive sleep apnoea syndrome; GERD: gastro-oesophageal reflux disease; FVC: forced vital capacity; DLCO: diffusion of the lung for carbon monoxide; 6MWT: six minute walking test; SpO2: peripheral oxygen saturation; mMRC: modified British Medical Research Council Questionnaire; GAP: Gender Age Physiology.
After one year of treatment, 57/133 (42.9%) patients showed stable/improved FVC values from baseline (i.e. ≥0% FVC ml). 30 (52.6%) patients had an increase of FVC (ml) from baseline of 0 to <5%, 9 (15.8%) between 5 and 9%, and 18 (31.6%) ≥10%. Median (IQR) increase was 110 (50–290) ml.
76 (57.1%) patients showed stable/improved FVC measured as percentage of the predicted value.
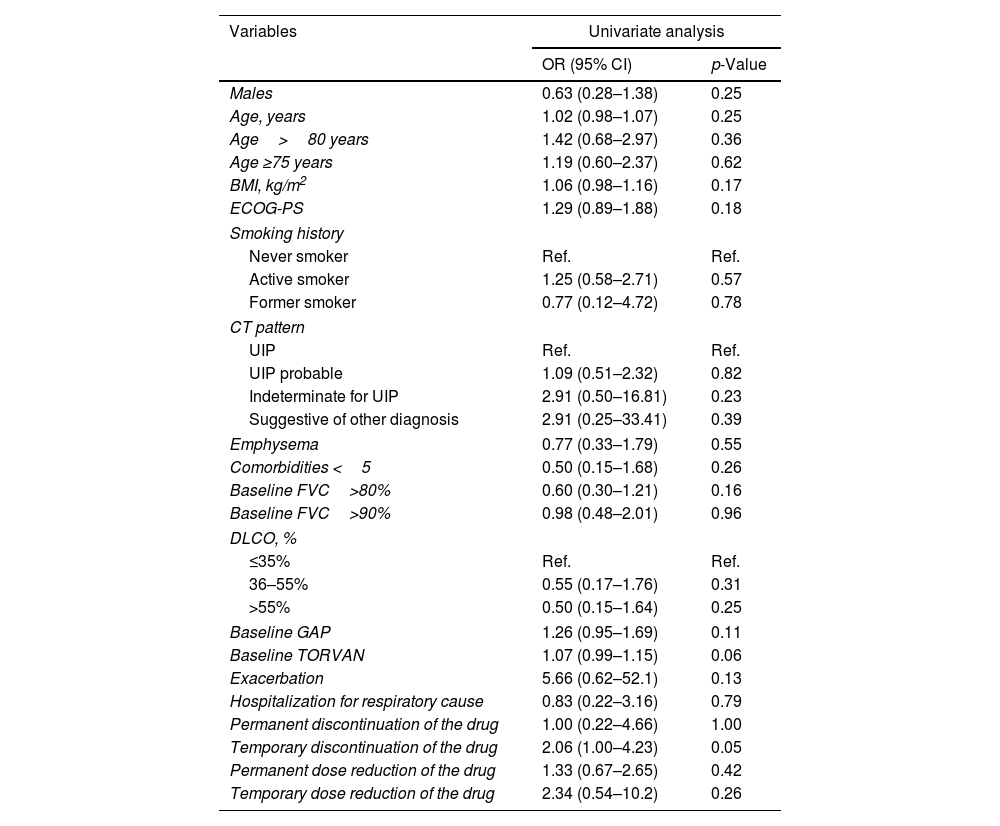
Univariate analysis did not show any predictors of FVC (ml) stability/improvement after one year of therapy (Table 2).
Logistic regression analysis to assess the relationship between clinical, functional, radiological and pharmacological variables and stability/improvement of FVC (ml) after one year of treatment with nintedanib.
| Variables | Univariate analysis | |
|---|---|---|
| OR (95% CI) | p-Value | |
| Males | 0.63 (0.28–1.38) | 0.25 |
| Age, years | 1.02 (0.98–1.07) | 0.25 |
| Age>80 years | 1.42 (0.68–2.97) | 0.36 |
| Age ≥75 years | 1.19 (0.60–2.37) | 0.62 |
| BMI, kg/m2 | 1.06 (0.98–1.16) | 0.17 |
| ECOG-PS | 1.29 (0.89–1.88) | 0.18 |
| Smoking history | ||
| Never smoker | Ref. | Ref. |
| Active smoker | 1.25 (0.58–2.71) | 0.57 |
| Former smoker | 0.77 (0.12–4.72) | 0.78 |
| CT pattern | ||
| UIP | Ref. | Ref. |
| UIP probable | 1.09 (0.51–2.32) | 0.82 |
| Indeterminate for UIP | 2.91 (0.50–16.81) | 0.23 |
| Suggestive of other diagnosis | 2.91 (0.25–33.41) | 0.39 |
| Emphysema | 0.77 (0.33–1.79) | 0.55 |
| Comorbidities <5 | 0.50 (0.15–1.68) | 0.26 |
| Baseline FVC>80% | 0.60 (0.30–1.21) | 0.16 |
| Baseline FVC>90% | 0.98 (0.48–2.01) | 0.96 |
| DLCO, % | ||
| ≤35% | Ref. | Ref. |
| 36–55% | 0.55 (0.17–1.76) | 0.31 |
| >55% | 0.50 (0.15–1.64) | 0.25 |
| Baseline GAP | 1.26 (0.95–1.69) | 0.11 |
| Baseline TORVAN | 1.07 (0.99–1.15) | 0.06 |
| Exacerbation | 5.66 (0.62–52.1) | 0.13 |
| Hospitalization for respiratory cause | 0.83 (0.22–3.16) | 0.79 |
| Permanent discontinuation of the drug | 1.00 (0.22–4.66) | 1.00 |
| Temporary discontinuation of the drug | 2.06 (1.00–4.23) | 0.05 |
| Permanent dose reduction of the drug | 1.33 (0.67–2.65) | 0.42 |
| Temporary dose reduction of the drug | 2.34 (0.54–10.2) | 0.26 |
BMI: body mass index; ECOG-PS: Eastern Cooperative Oncology Group-Performance Status; CT: computed tomography; UIP: usual interstitial pneumonia; FVC: forced vital capacity; DLCO: diffusion of the lung for carbon monoxide; GAP: Gender Age Physiology.
This is to our knowledge the first observational study investigating variables related to a stability/improvement of FVC in patients with IPF after one year of treatment with nintedanib.
We did not find any clinical, functional, radiological, and pharmacological variable significantly associated with disease stability or improvement. These findings confirm the unpredictable course of IPF, also during antifibrotic treatment.
IPF has been historically considered the prototype of progressive pulmonary fibrosis (PPF), but data from experimental and real-life studies highlighted that a subgroup of patients shows a stabilization of FVC during the first year of antifibrotic treatment.1,4–6 The mechanisms by which nintedanib may lead to a functional improvement are unknown.
A previous post hoc analysis of INPULSIS trials showed a stability/improvement of FVC (ml) in 24.8% of patients treated with nintedanib VS. 9% treated with placebo.4 The Authors failed to detect baseline clinical and functional predictors of FVC stability or increase.4 However, variables related to comorbidities, exacerbations and treatment, which may potentially influence the course of the disease and survival in patients with IPF, were not assessed.
Our study confirms previous findings and failed to detect any significant impact of hospitalizations, exacerbations, comorbidities, drug discontinuation, and dose reduction on the outcome. The observational nature of our study might explain the numerical discrepancy with the findings of the clinical trials.4
Other observational studies demonstrated a relative stability of FVC over the first year of treatment.5,7,8
In our study, 42.9% (milliliters) and 57.1% (percentage of the predicted value) of patients who completed one year of follow-up showed stability/improvement of FVC values.
Our findings are consistent with a recent observational Italian study that found a 47.4% of stability/improvement of FVC percentage predicted in 133 patients after 12 months of nintedanib, with similar proportion of patients among FVC variation classes.5
Moreover, results from the Canadian Registry for Pulmonary Fibrosis (CARE-PF) showed that only 59% of IPF patients during antifibrotic treatment met the pragmatic criteria of progression over a 24-months interval.9
St George's Respiratory Questionnaire (SGRQ) was suggested as a sensitive tool to detect FVC changes in patients with IPF, but no significant changes were found in non-decliners recruited in INPULSIS trials.4,10 SGRQ score was not included in our retrospective study since it is not routinely adopted in our daily clinical practice.
Some study limitations should be acknowledged. The retrospective nature of the study could affect the reliability of the findings which should be confirmed in a larger cohort and over a longer timeframe.
In conclusion, a significant proportion of patients treated with nintedanib shows a stability/improvement of FVC after one year of treatment but no clinical, functional, radiological and pharmacological variables were associated with a positive prognosis.
Future prospective studies are needed to confirm our findings over a longer period, as well as to assess if genetic and/or serological variables may predict disease treatment response in PPF.
Authors’ contributionsMichele Mondoni: conceptualization; investigation; supervision; writing – original draft; Francesco Varone: conceptualization; investigation; writing – original draft; Fausta Alfano: investigation, writing –review and editing; Giuseppe Muscato: investigation, writing – review and editing; Caterina Conti: investigation; writing – review and editing; Bruno Iovene: investigation, writing – review and editing; Laura Saderi: formal analysis; writing – review and editing; Fabiano Di Marco: investigation, writing – review and editing; Stefano Centanni: investigation; supervision; writing – review and editing; Carlo Vancheri: investigation; writing – review and editing; Luca Richeldi: investigation; writing – review and editing. Giovanni Sotgiu: formal analysis; methodology; supervision; writing – original draft.
Role of the sponsorsNone.
Conflict of interestMichele Mondoni received fees for lectures from Boehringer Ingelheim (outside the present work); Fausta Alfano received fees for lectures from Boehringer Ingelheim (outside the present work); Francesco Varone received fees for lectures from Boehringer Ingelheim (outside the present work); Caterina Conti received fees for lectures from Boehringer Ingelheim (outside the present work); Fabiano Di Marco received fees for lectures from Boehringer Ingelheim (outside the present work); Stefano Centanni received fees for lectures from Boehringer Ingelheim (outside the present work); Carlo Vancheri: received fees for lectures from Boehringer Ingelheim (outside the present work); Luca Richeldi: received fees for lectures from Boehringer Ingelheim (outside the present work).
Giuseppe Muscato, Bruno Iovene, Laura Saderi and Giovanni Sotgiu have no conflict of interest to disclose.
Guarantor statement, Michele Mondoni is the guarantor of the content of the manuscript, including the data and analysis.