SARS-CoV-2 infection has caused more than 5 million deaths worldwide and has had a major impact on the economy and healthcare. Vaccination has changed the management of these patients by preventing transmission and reducing mortality.1
Side effects associated with SARS-CoV-2 mRNA vaccines, some serious, have been reported. One of the major adverse events associated with the Moderna vaccine (mRNA-1273), myocarditis, seen primarily in young adults, occasionally occurs concomitantly with heart disease, while another rarely reported event is multisystem inflammatory syndrome (MIS).2
We report a clinical case of MIS following administration of the Moderna vaccine. To our knowledge, this is the only published case of pleural exudate in this context.
Our patient was a 33-year-old woman, with no drug allergies, smoker of 5 pack-years, with no medical or surgical history of interest. She was not receiving any regular treatment, and worked at home as a mother of 2 children with a dog and a cat as pets.
She attended the emergency room with a 3-week history of sternal pain that worsened with postural changes. The symptoms had developed 5 days after administration of the second dose of the Moderna vaccine. She also reported moderate dyspnea on exertion, low-grade fever, and arthralgias, with no cough or other symptoms.
On physical examination, she was in good general condition, with normal coloring, normal hydration, and normal breathing at rest. She had no supraclavicular lymphadenopathies or nail clubbing. Blood pressure was 101/70mmHg, heart rate 74bpm, respiration rate 12 breaths/m, and SpO2 (room air) 98%
Cardiac auscultation was normal and pulmonary auscultation showed decreased breath sounds in the lower third of both lungs, with decreased transmission of vocal fremitus, dullness on percussion, and signs of pleural effusion. The rest of the examination was normal.
Antigen and polymerase chain reaction (PCR) for COVID-19 were negative.
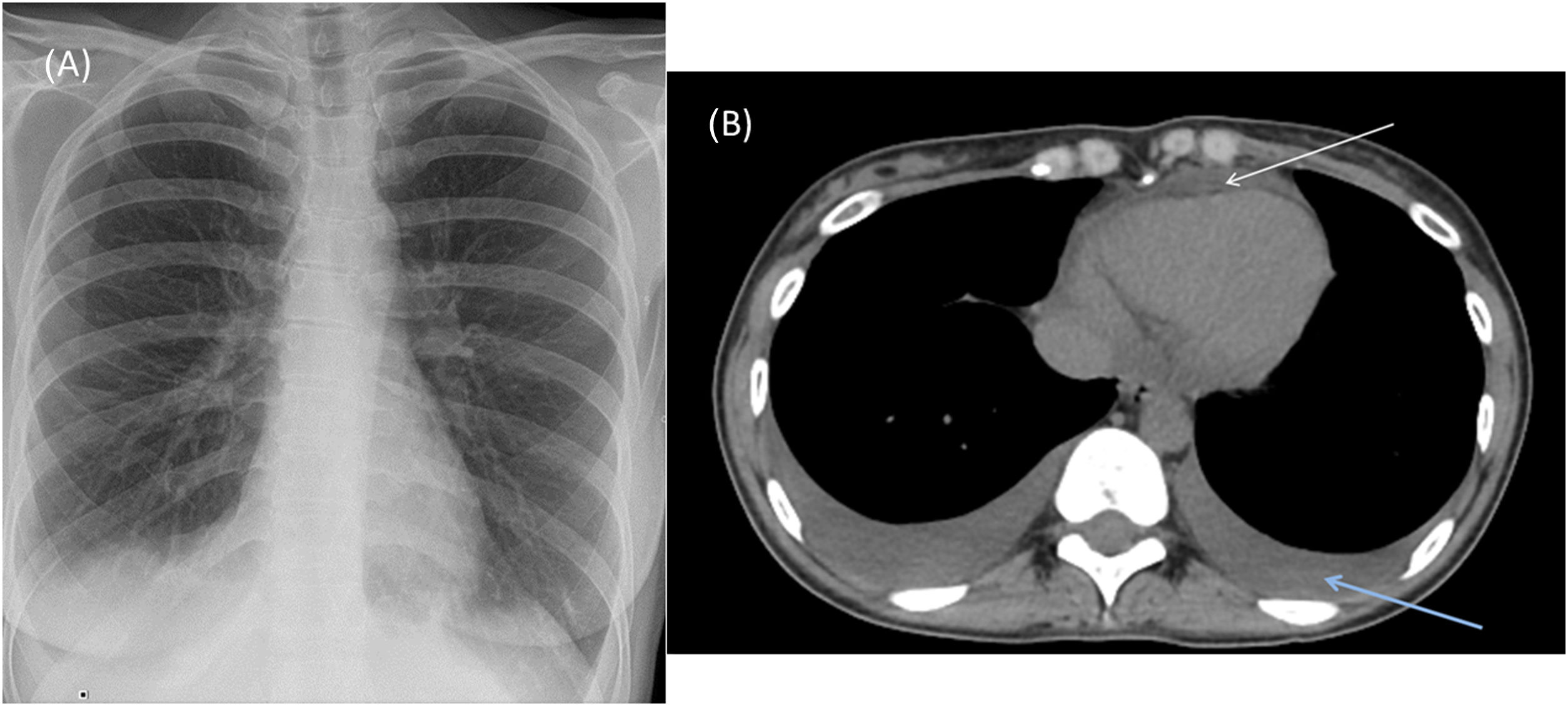
Chest X-ray in the emergency room showed blunting of both costophrenic angles consistent with bilateral pleural effusion (Fig. 1A).
(A) Anterior-posterior chest X-ray at admission showing right pleural effusion and left costophrenic angle blunting. (B) Chest computed axial tomography showing bilateral pleural effusion and pericardial effusion (upper arrow indicates pericardial effusion and the right arrow indicates pleural effusion).
The blood count showed 10.1×109/l leukocytes with 5.9% eosinophils (600eosinophils) and hemoglobin 14.2g/dl. Biochemistry showed total proteins 6.69g/dl, LDH 257U/l, CRP 4.03mg/dl. Coagulation tests showed D-dimer 2656ng/ml.
Pneumococcus and Legionella antigens in urine and parallel respiratory serology for Legionella, Mycoplasma and respiratory viruses were negative.
A chest computed tomography angiogram conducted to rule out pulmonary thromboembolism confirmed moderate bilateral pleural effusion with bibasilar and middle lobe atelectasis (Fig. 1B). This was confirmed on chest ultrasound that also ruled out vascular involvement.
Echocardiography showed mild pericardial effusion not affecting cavity filling. Abdominal ultrasound was normal.
Thoracentesis showed exudate (proteins 4.25g/dl and LDH 191U/l) with predominantly polymorphonuclear cells (73.2%), normal ADA (6.7U/l), cholesterol 75mg/dl, CEA<1.7ng/ml, and negative antinuclear antibodies. Cytology was negative for malignancy, culture showed no microorganisms, and Ziehl-Neelsen and culture in Löwenstein-Jensen media were negative.
Azithromycin 500mg/24h and methylprednisolone 40mg/12h were administered for 6 days with favorable clinical and radiological evolution, and the patient was discharged with prednisone 30mg in a tapering regimen and azithromycin 500mg/24h for a further 4 days, and was referred for monitoring in the outpatient clinic.
Follow-up chest CT at 15 days showed a marked decrease in pleural and pericardial effusion.
Follow-up clinical laboratory tests at 23 days after discharge showed normal antinuclear antibodies and anti-neutrophil cytoplasm antibodies (P-ANCA and C-ANCA). Serology was positive for SARS-CoV-2 IgG.
The Moderna mRNA-1273 vaccine is given in 2 doses and has demonstrated 94.7% efficacy and very good safety. Most of the adverse effects are mild, and while serious events, such as myalgias, arthralgias and asthenia, have been reported, particularly after the second dose, pleural and pericardial effusion are not listed in the Summary of Product Characteristics or reported in the literature.3
Our patient, who had recently received the Moderna vaccine, developed bilateral and pericardial pleural effusion. After ruling out other causes, her clinical picture was consistent with MIS caused by the Moderna vaccine, resulting in bilateral pleural and pericardial exudate.
MIS after vaccination (usually the second dose) is exceptional, and very few cases have been published, particularly in children and adolescents. It consists of a systemic clinical syndrome (diarrhea, dyspnea, abdominal pain, skin rash, and hypotension) involving at least 2 organs. Progress is favorable after administration of corticosteroids and, in some cases, intravenous immunoglobulins.4
This entity may have clinical similarities with active SARS-CoV-2 infection, so assessment of the vaccination history is essential when taking the medical history. Most cases are reported at 2–4 weeks after administration and involve gastrointestinal symptoms. The only cases of pleural effusion reported have been associated with heart failure, and none of these patients were studied for pleural fluid. Long-term functional consequences are unknown.5
The SARS-CoV-2 vaccine – generally the Janssen vaccine – has been associated with some rare reports of MIS in the literature, and in no case has pleural fluid been studied to complete the diagnosis. Exudative pleural-pericardial effusion without accompanying myocarditis should be considered among the complications caused by the mRNA-1273 vaccine.