Tuberous sclerosis complex (TSC) is an autosomal dominant syndrome characterized by mental deficiency, epilepsy and skin lesions (angiofibromas and subungual fibromas).1 Lung affectation appears in 1%–2.3% of patients with TSC, mainly in the form of lymphangioleiomyomatosis (LAM),1 although recent studies suggest that radiological findings may be seen in 26%–39% of patients.2 Multifocal micronodular pneumocyte hyperplasia (MMPH) is the second lung manifestation and extremely rare. It may appear associated with LAM or, less frequently, as an isolated lung affectation in pre- or post-menopausal women with TSC, but it has also been reported in men with or without TSC.3,4
In reviewing the literature (Medline, 1991–2012), we have not found any publications in Spanish, and there are less than 50 cases reported with lung biopsy. We present the case of a 21-year-old woman diagnosed since childhood with TSC, with no known family history, and autoimmune hypothyroidism with substitutive treatment. Physical exploration was normal. Baseline arterial oxygen saturation was 98%. Basic analytical parameters were within normal limits, except for: urea 108mg/dl; creatinine 3.7mg/dl; urate 7.7mg/dl, and pH in venous blood 7.29.
Cerebral magnetic resonance imaging showed subependymal nodules and lesions in the bilateral occipital and left parietal white matter, with persistence of astrocytomas. Chest radiography was done during the study prior to renal transplantation, which showed a bilateral nodular interstitial pattern, and the patient was therefore referred to the pulmonology department. The patient reported no occupational or leisure risks, nor did she present respiratory symptoms at that time.
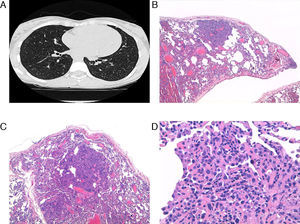
Computed tomography (CT) demonstrated a bilateral nodular pattern of between 1 and 8mm in diameter (Fig. 1), with no cystic lesions. In previous studies done during childhood, lung parenchyma was normal. Spirometry values were within normal limits, except for a slight reduction in carbon monoxide diffusion capacity.
(A) Micronodular pattern seen on computed tomography slice. (B and C) Parenchyma with altered architecture and areas of increased cell density and pseudo-nodular appearance (HE, ×40 and ×100). (D) Detailed view of the lesion, with increased septal thickness, pneumocyte hyperplasia and alveolar collapse (HE, ×200).
Six months later, CT was repeated and the same pattern persisted, so we decided to perform lung biopsy with video-assisted thoracoscopy. In the lung biopsy, macroscopically there were whitish nodular areas observed, measuring 2–3mm. Histology detected lung parenchyma with altered architecture where areas were observed with increased cell density with pseudo-nodular appearance, and among which there was normal lung parenchyma. The areas with pseudo-nodular appearance were located at the peripheral and central levels and constituted by lung parenchyma with thickening of septa that were often collapsed and covered with hyperplastic type II pneumocytes, with cubic morphology. There was no mitosis, necrosis, cystic lesions or proliferation of immature muscle cells suggestive of LAM (Fig. 1), all of which was compatible with the diagnosis of MMPH.
TSC is an autosomal dominant syndrome, but in up to one-third of cases there is no family history, as in the case we provide.1 MMPH was described for the first time by Popper et al.4; it is generally associated with LAM, but it has also been described as a the only lung affectation in women and men with TSC, and more rarely without TSC.3,4 Clinical manifestations include dry cough, moderate-exertion dyspnea, moderate or asymptomatic hypoxemia.1,3,5 The radiological alterations of MMPH are not specific, and the differential diagnosis should include pulmonary tuberculosis, sarcoidosis, histiocytosis X, tumorlets and pulmonary metastases. The definitive diagnosis is established by lung biopsy. In small samples it is particularly difficult to differentiate it from papillary adenoma. The natural history is not clear, but it is unlikely for it to degenerate to malignancy, and the clinical evolution is good although there have been reported episodes of death due to respiratory failure.3,5,6
Please cite this article as: Miravet Sorribes L, et al. Hiperplasia micronodular neumocitaria multifocal en una paciente con esclerosis tuberosa. Arch Bronconeumol. 2012;49:36–7.