Vaccination is the most effective measure in the prevention of invasive pneumococcal disease (IPD). High-risk patients immunized during medical visits would benefit from the vaccine.
ObjectivesTo describe the IPD cases. To assess the most prevalent causative serotypes and to evaluate the missed opportunities for vaccination.
MethodsThis is a descriptive retrospective study of the incidence of IPD cases in Elche during 5 years. The vaccination status and the visits to specialized care prior to disease were reviewed. The effectiveness with the 23-valent pneumococcal vaccine in our population was also calculated.
ResultsBetween 2007 and 2011, 181 cases of IPD were notified; the most frequent medical conditions were pneumonia and sepsis, with a mortality rate of 12%. 80% of the causative serotypes are included in the vaccine. More than the half of the cases had at least one of the risk factors for indicating the vaccination. This percentage decreases by 6.2% in cases below 65 years of age with any risk factor.
ConclusionsAfter 10 years of introducing the vaccine into the adult immunization schedule the coverage is still low among the patients with risk factors. In our study, 75% of the cases were not vaccinated. Taking in count the vaccine effectiveness for preventing IPD, among the patients attended at the hospital by the specialist prior their IPD, it could have been prevented in the best assumption (85% vaccine effectiveness) 60 IPD cases.
La vacunación es la medida más efectiva para la prevención de la enfermedad neumocócica invasiva (ENI). Los pacientes con enfermedades predisponentes se podrían beneficiar de esta vacuna si se recomienda durante las visitas médicas.
ObjetivosDescribir los casos de ENI. Valorar los serotipos más frecuentes y evaluar oportunidades perdidas de vacunación.
MetodologíaEstudio descriptivo retrospectivo de la incidencia de ENI en Elche durante 5 años. Se ha revisado el estado vacunal y las visitas a atención especializada previas a la enfermedad. También se ha calculado la efectividad vacunal con vacuna antineumocócica 23 valente en nuestra población.
ResultadosDesde 2007 a 2011 se han notificado 181 casos de ENI. Las formas clínica más frecuentes son neumonía y sepsis, con una tasa de mortalidad del 12%. El 80% de los serotipos causales son serotipos vacunales. Más de la mitad tenían alguno de los factores de riesgo que indican vacunación. Este porcentaje disminuye hasta el 6,2% en los menores de 65 años con algún factor de riesgo.
ConclusionesTras 10 años de introducción de la vacuna en el calendario vacunal del adulto sigue siendo baja la cobertura vacunal de pacientes con factores de riesgo. En nuestro estudio, el 75% de los casos no están vacunados. Teniendo en cuenta la efectividad de la vacuna para la prevención de ENI, entre los pacientes que han sido atendidos en el hospital por el especialista previo a su ENI, se podrían haber prevenido en el mejor de los supuestos (85% de efectividad vacunal) 60 casos de ENI.
Invasive pneumococcal disease (IPD) is a frequent cause of mortality in older adults, and contributes significantly to excess morbidity and hospitalization in the rest of the population. As such, IPD is considered a major public health problem in Spain.1,2
IPD is less common than non-invasive pneumococcal pneumonia, but the presence of Streptococcus pneumoniae (pneumococcus) in sterile fluids is associated with a higher fatality rate. Over 90 different serotypes of this organism have been identified worldwide, but only 20 serotypes account for over 70% of invasive pneumonia cases in all age groups.3
Since these serotypes are covered by pneumococcal vaccines, a prevention measure benefitting at-risk patients might be the medical advice given during office visits by health professionals recommending pneumococcal immunization.4,5
In the year 2000, vaccination with 23-valent polysaccharide pneumococcal vaccine (VP23) was indicated in the Region of Valencia, Spain, for risk groups,6 including patients >65 years of age, patients with asplenia (functional or organic) and AIDS patients. Indications for other risk groups have subsequently been extended to include heart disease, chronic respiratory diseases, diabetes mellitus, and other immunodeficiencies.
In 2003, the Ministry of Health issued an order defining IPD as a notifiable disease (ND), with the aim of achieving a better understanding of its incidence, and IPD was thus incorporated in the epidemiological surveillance system. These reports have been notified electronically in Valencia since 2007 (AVE system).
In this region, the IPD rate has remained stable, with an average rate of 17.8 cases per 105 inhabitants over the last five years.5 Therefore, the number of cases remains high, despite the introduction of immunization of risk groups and the recommendation of pediatric immunization with conjugate vaccines7 (not yet included in the pediatric immunization schedule).
Although the value of vaccination as a preventive measure of disease is scientifically proven, the effectiveness and long-term efficacy of this vaccine are a source of controversy among health professionals in many forums,8–10 and there is some justified doubt regarding the compliance of these professionals with the indications for vaccination.11,12
Two types of pneumococcal vaccines are currently available: VP23, that protects against 23 serotypes and is indicated in the vaccination schedules for the adult population and the at-risk population from 2 years of age; and the more modern conjugate vaccines (serotypes 10 and 13), indicated for children and recently approved for use in adults older than 50 years. Conjugate vaccines are not included in the pediatric schedule, but their use is recommended by primary care pediatricians.
The purpose of this study was to analyze IPD cases reported by way of the AVE system to the Health Department of Elche in the Region of Valencia, to identify the most common pathogenic serotypes in this environment, to describe the vaccination status in IPD cases, and to test the effectiveness of immunization with VP23. Another aim was to assess missed opportunities in IPD cases.
MethodsThis is a retrospective study of the incidence of IPD in the Health Department of Elche, which serves a population of approximately 260 000 inhabitants.
An electronic record of all ND was created in Valencia under the name of Sistema de Análisis de Vigilancia Epidemiológica (AVE, Analysis System for Epidemiological Surveillance), in which each ND is electronically recorded. Thus, the Health Departments can easily retrieve the information recorded in this system. We selected all IPD cases reported in the AVE system from January 2007, the date on which electronic recording started, until December 2011, both inclusive.
According to this reporting protocol, a confirmed case of IPD is a patient presenting with symptomatic infection and pneumococci isolated in a normally sterile biological sample culture (blood, pleural fluid or spinal fluid).
The variables age, gender, type of pneumococcal disease, previous diseases and other risk factors were retrieved from all reported cases, in order to determine whether there was a need for prior vaccination. Risk factors were determined from the categories of medical conditions for which vaccination is indicated, according to the Advisory Committee on Immunization Practices.13,14 Microsoft Excel 2003 was used to build the study database and perform the statistical analysis.
Additionally, immunization status was assessed in each case with a personal questionnaire, immunization card records, and the nominal immunization registry, the official database in which any vaccination event is electronically recorded. This database is linked to the individual health identification document and the electronic medical record of each patient, and registers the event with details on the type of vaccine administered, the date of administration and the dose.
Data from the last population census conducted by the Spanish National Institute of Statistics (Instituto Nacional de Estadística)15 were used to calculate rates per population.
Vaccine effectiveness (VE) was calculated taking into account only cases of patients older than 2 years, in whom VP23 can be administered according to technical specifications and the vaccination schedule. VE was calculated using the self controlled case series method with the Farrington formula: VE=(PVP−PVC)/PVP (1−PVC),16,17 where PVC is the proportion of vaccinated cases and PVP is the proportion of the vaccinated population.
As official statistics on vaccination coverage according to risk groups were not available, PVP was estimated from a consecutive sample of all patients admitted to hospital during the first 6 months of 2010, whose primary or secondary diagnosis in the discharge report had been medical conditions considered indications for vaccination according to the Advisory Committee on Immunization Practices (e.g., COPD, diabetes mellitus, chronic heart failure). Subsequently, they were grouped into the same risk categories used in the epidemiological survey (chronic heart disease, chronic lung disease, cirrhosis, chronic otitis, cochlear implant, diabetes mellitus, asplenia and immunodeficiencies). The statistical application EPIDAT 3.1 was used to calculate VE and confidence intervals at 95%.
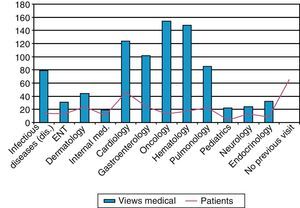
Additionally, the database registry of hospital and specialized activity (HIGUIA) was analyzed, starting in the year 2000, to detect all visits to specialty care by patients older than 2 years that had taken place before their IPD. All visits to any outpatient medical specialty in the following departments were counted: Gastroenterology, Pulmonology, Internal Medicine, Infectious Diseases, Hematology, Oncology, Nephrology, Endocrinology, Cardiology, Pediatrics and Otolaryngology. We defined as “missed opportunity for IPD vaccination” any case that met the following criteria: (1) patient not vaccinated with VP23 and (2) who had made at least one visit before IPD to a hospital consultant in the selected medical specialties.
ResultsBetween January 1, 2007 and December 31, 2011, a total of 181 cases of IPD were notified via the AVE system in the Elche Department of Health. Average IPD rate for five years is 17.8 cases per year per 105 inhabitants. There was a peak in 2010 of 28.3 cases per 105 inhabitants which coincided with the epidemic of influenza A (H1N1)n.
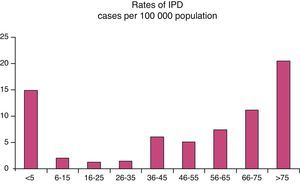
No significant differences were found for gender. As for age, 62% were under 65 years (113 patients) (Fig. 1).
All (100%) of the studied cases required hospitalization. Of these, 92.1% had symptoms suggestive of pneumococcal disease and the most common clinical manifestation was pneumonia (66%).
Regarding the existence of predisposing risk factors for IPD, the results were as follows: 27% had chronic lung disease, 25% had a diagnosed chronic cardiovascular disease, 16% had diabetes mellitus, 4% chronic otitis, 3% had a previous head injury, 5% were HIV positive, and 20% had another immunodeficiency. No risk factor was found in a third of cases (33%), including age over 65 years as a risk factor (Table 1). Thirty-three cases had more than one risk factor.
The fatality rate was 12%, and more than half of the deaths occurred in patients older than 65 years. In this group, the mortality rate was 22%.
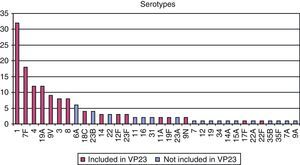
Regarding the microbiological study of strains obtained in sterile fluids, pneumococcus was isolated in 85% of samples and, of these, serotyping was possible in 119 cases. A serotype included in the adult-vaccine (VP23) was found in 79% of serotyped samples (Fig. 2).
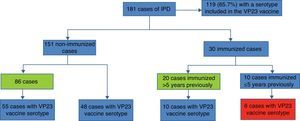
The classification of cases by immunization status before an IPD episode revealed that 75% were unvaccinated (Fig. 3). Of these, nearly 60% had a risk factor indicating vaccination, so these were considered preventable cases. Six patients vaccinated less than five years before having IPD with a serotype included in the VP23 vaccine were considered immunization failure.
Finally, in order to assess whether there was a prior indication for vaccination in unvaccinated cases, we screened the previous consultations of patients older than 2 years in any of the risk groups for vaccination that had been seen in specialized units since the pneumococcal vaccination protocol was published in 2000. These revealed a total of 864 consultations, with an average of 11 previous visits per patient. Sixty-five (65) cases (36%) had not presented in any of the selected specialist departments (Fig. 4).
As for VE in our population, using Farrington's formula, we have calculated the effectiveness of VP23, taking into account only the serotypes included in the vaccine, and the cases vaccinated in the last 5 years. The results are shown in Table 2.
Effectiveness of Vaccination by Risk Group.
| Risk group | Population | Cases | Vaccination effectiveness | |||||||
| n | PVP | 95% CI | n | PVC | 95% CI | Serotypes VP23 | 95% CI | <5 years | IC 95% | |
| Chronic heart disease | 1245 | 46.7 | 44–49 | 27 | 22.2 | 9–42 | 67 | 20–87 | 57 | −5–83 |
| Chronic lung disease | 669 | 43.4 | 40–47 | 33 | 18.2 | 7–35 | 71 | 31–88 | 83 | 44–95 |
| Cochlear implant | 55 | 18.2 | 7–29 | 0 | 0 | – | –a | –a | ||
| Chronic otitis | 23 | 26.1 | 10–48 | 4 | 0 | 0–60 | –a | –a | ||
| Diabetes mellitus | 1690 | 39.8 | 37–42 | 19 | 21.1 | 6–46 | 58 | –20–87 | 68 | −8–91 |
| Immunodeficiencies | 753 | 45.3 | 42–49 | 25 | 28.0 | 12–49 | 52 | –13–80 | 78 | 27–93 |
| Total | 4435 | 42.9 | 41–44 | 119 | 13.4 | 8–21 | 79 | 65–88 | 85 | 71–92 |
Ten years after the introduction of the pneumococcal vaccine in the adult immunization schedule, vaccine coverage in our country remains poor. In the population sample used to calculate vaccine effectiveness, vaccination coverage does not exceed 50% in any of the risk groups. Previous studies, such as that of Pebody et al. in England and Wales, found population coverage in similar risk groups to be 69% for cochlear implant, 53.4% for splenic dysfunction, 36.5% in chronic cardiovascular disease, 34.7% diabetes, 22.9% immunosuppression, 28.7% chronic kidney disease, 15.9% sickle cell disease, and 12.6% in chronic lung disease.18 Lu and Nuorti, in a study from the CDC, found 26.1% vaccination coverage for all risk groups of workers between 18 and 64 years.19
In our study, we found that 81.7% of IPD cases occurred in unvaccinated patients who had a previous indication for vaccination, belonging to risk groups recognized as susceptible to an invasive form of pneumococcus. This confirms two issues: on one hand, the failure to vaccinate subjects with diseases already recognized as a risk factor, and on the other, the higher incidence of pneumococcal disease in these risk groups.
As for pneumococcal invasive infection in subjects with no risk factors, 59 people under the age of 65 years in our population with no medical record of underlying disease presented IPD.
Regarding the most accepted indication for vaccination, age over 65 years, 65% of cases with invasive disease and an age above 65 years were not vaccinated. A joint vaccination campaign was carried out in 2001 during the influenza vaccination campaign, accompanied by an information campaign aimed at health centers from the Spanish General Directorate of Public Health for the promotion of vaccination in this group. However, this indication was not emphasized in later years due to the difficulty of knowing whether the patients had been previously immunized. For instance, in 2011 55 people were vaccinated due the age risk factor, while the population reaching 65 years of age during 2011 was nearly 1500. In USA, vaccination coverage in this group is 60%,20 far from the goal of achieving 90% vaccination coverage in the elderly.
Regarding the effectiveness of immunization with 23-valent vaccine in our population, we found that overall vaccine effectiveness is 85% for the serotypes included and individuals vaccinated within five years. This rate is higher than that reported in other papers: VE was 55% in the 2008 Cochrane Review4; between 56 and 92% in controlled clinical trials; and between 48 and 81% in non-randomized experimental studies.21 Possibly, careful case selection, including microbiological diagnosis from a normally sterile sample, might have increased VE, since less severe forms of the disease in which no specific diagnostic techniques were performed were excluded.
It is also possible that selection of the sample for calculating vaccination coverage in risk groups increased VE, as the vaccination rate in the population was determined from at-risk patients who were admitted to hospital; since their underlying disease was more severe, the likelihood of being vaccinated may have been higher.
As for the lower vaccine effectiveness in the immunocompromised risk group, the findings of this study are in line with those obtained in others.7,22
It is remarkable, considering that 80% of the isolates cultivated were vaccine serotypes, that there is a failure to prevent this disease. In this respect, one of the main goals of our study was to screen possible missed opportunities for vaccination in patients with IPD. In 71 unvaccinated at-risk patients, we found at least one occasion during which vaccination might have been recommended. This means that, with a vaccine efficacy of 85%, 60 cases of IPD might have been prevented. With regard to cases with chronic respiratory disease (49 patients), eight of them had seen a pulmonologist, meaning that with a specific VE of 83% for this group, 6 cases of IPD might have been prevented.
In the community under study, vaccination with VP23 according to the vaccination schedule (age over 65) is carried out in primary care. In the hospital setting, the Preventive Medicine Unit is responsible for carrying out vaccination in at-risk patients, and these patients should be referred by their treating specialists. A study conducted in a hospital in Madrid found a significantly better level of knowledge among primary care physicians than among specialized care physicians.21 An important finding of that study is that less than a third of the hospital staff said that they explicitly recommend vaccination to their patients. These data could not be evaluated in our hospital, but the impression of the authors is that many patients do not receive recommendation for vaccination, as confirmed by the data presented in this report. This highlights a need for raising awareness, informing, recommending and promoting vaccination among health professionals working in the hospital setting. In mid-2011, a series of informative meetings with specific departments were initiated, aimed at reinforcing the information on immunization received by specialists who care for patients in risk groups. The effectiveness of this measure has not yet been evaluated, but the hope is that it will lead to a decline in the rate of IPD in our adult population.
As for the observed vaccine failures, three occurred in patients with severe immunodeficiency, underlining the poor immune response to the vaccine in this population. However, the sample is small and conclusive data cannot be drawn.
ConclusionsThe authors believe that an informational strategy should be directed at specialist physicians in the hospital setting, reiterating the indications for vaccination in patients with predisposing risk factors. This might improve the current vaccine coverage in adults, and may well produce a decline in invasive pneumococcal disease forms. It may also introduce into clinical practice new indications for the 13-valent conjugate vaccine in adults, especially in cases with reduced immune response to the polysaccharide vaccine.23–25
FundingThe authors have not received funding for conducting this study.
Authors’ ContributionsMercedes Arencibia Jiménez, Consultant in Preventive Medicine and lead researcher, conducted the literature search, statistical analysis of the data and drafted the paper.
Juan Francisco Navarro Gracia, Head of Preventive Medicine, supervised the study and contributed with his expertise.
Jose Antonio Delgado de los Reyes and Gerardo Pérez Torregrosa, Preventive Medicine resident physicians, carried out the epidemiologic surveys of IPD cases and checked the vaccination status of individual cases and the population.
David López Parra and Pilar López García, resident physician and attending physician in Microbiology respectively (Microbiology Department), carried out the isolation and serotyping of pneumococcus strains.
Conflicts of InterestThe authors declare no conflict of interest in the development of this work.
We thank the Epidemiological Surveillance Department of the Elche Department of Health (Servicio de Vigilancia Epidemiológica del Departamento de Salud de Elche) for their permission to use the information collected through the notifiable diseases surveillance system.
Please cite this article as: Arencibia Jiménez M, Navarro Gracia JF, Delgado de los Reyes JA, Pérez Torregrosa G, López Parra D, López García P. Oportunidades perdidas de vacunación antineumocócica. ¿Se puede hacer algo más en prevención? Arch Bronconeumol. 2014;50:93–98.