Pulmonary arterial hypertension is characterized by obstruction of the pulmonary arteries. The gene, mainly related to pathology, is the bone morphogenetic protein receptor type II (BMPR2). The aim of this study was to analyze the methylation pattern of the BMPR2 promoter region in patients and controls.
MethodsWe used Methyl Primer Express® v.1.0 and MatInspector softwares to analyze this region. Genomic DNA obtained from the peripheral blood of patients and controls was modified with sodium bisulphite. Methylation was analyzed using methylation-specific PCR. DNA treated with CpG methyltransferase was used as a positive control for methylation and H1299 cell culture DNA was used as positive control for gene expression.
ResultsWe identified a CpG island, which may have been methylated, in the BMPR2 promoter region, in addition to NIT-2 (global-acting regulatory protein), sex-determining region Y and heat shock factor transcription factor binding sites. We found no evidence of methylation in patients and controls. No methylated CpG sites were identified in H1299 cells expressing the BMPR2 gene.
ConclusionsThe BMPR2 promoter region is the most suitable for study because of the high number of transcription factor binding sites that could alter gene function. No evidence of methylation was detected in this region in patients and controls.
La hipertensión arterial pulmonar es una enfermedad infrecuente caracterizada por una obstrucción progresiva de las arterias pulmonares. El receptor tipo II de la proteína ósea morfogenética (BMPR2) es el principal gen relacionado con esta enfermedad. EL objetivo de este trabajo fue analizar el patrón de metilación de la región promotora del gen BMPR2 en individuos con hipertensión arterial pulmonar y controles.
MétodosSe analizó la región a estudio con los softwares Methyl Primer Express® v.1.0 y MatInspector. El ADN genómico, obtenido a partir de sangre periférica, fue modificado con bisulfito de sodio. La metilación se analizó utilizando PCR específica de metilación. Como control positivo de metilación se utilizó ADN tratado con CpG metiltransferasa y como control positivo de expresión se utilizó ADN del cultivo celular H1299.
ResultadosSe ha identificado una isla CpG susceptible de presentar metilación en la región promotora del gen que contiene secuencias específicas como sitios de unión a factores de trascripción NIT-2 (global-acting regulatory protein), sex-determining region Y y heat shock factor. No se han encontrado indicios de metilación en los pacientes ni en los controles. En las células H1299, que expresan el gen BMPR2, no se ha identificado metilación en la isla CpG analizada.
ConclusionesNo se detectaron indicios de metilación en la región promotora a estudio en pacientes y controles, siendo esta la región más adecuada para realizar el estudio debido al alto número de sitios de unión a factores de transcripción que podrían estar involucrados en la correcta funcionalidad del gen.
Pulmonary arterial hypertension (PAH; OMIM #178600; ORPHA 422) is a progressive disease characterized by the obstruction of the pulmonary arteries.1 It is defined by a sustained increase in mean pulmonary arterial pressure (mPAP)≥25mmHg at rest.2 Symptoms include dyspnea, syncope, chest pain, and, ultimately, premature death due to right heart failure.1 Structural and functional changes in the vascular wall and clot formation are the main factors causing pulmonary vascular resistance (PVR).3 PAH can be familial (FPAH) or idiopathic (IPAH), or it can be associated with other diseases, medications and toxic substances (APAH).4 It is more common in women than men, at a ratio of 1.7:1.2
Until recently, the genetic basis for PAH has been mainly associated with coding mutations in the bone morphogenetic protein receptor type II (BMPR2) gene. This gene is located on the 2q33 chromosome and mutations have been identified in over 80% of patients with FPAH. However, only 20% of carriers of this genetic mutation develop the disease.5,6 In patients with IPAH, BMPR2 mutations occur less frequently, and are found in smaller patient cohorts (6%–40%).6–9 Since the identification of this gene as the main cause of PAH, others have also been associated with the disease9 and some genetic modulators that can modify patient phenotypes have been identified, for example SLC6A4,10EDN1,11NOS2,12AGTR1 and TRPC6.13
DNA methylation is a modification which occurs in numerous organisms.14–17 Methylation in the promoter region of a gene induces transcriptional silence.15,18,19 Promoters associated with these CpG islands reveal multiple transcription initiation sites dispersed throughout the island20 which often act as bidirectional promoters.21–23
BMP proteins are signaling molecules which belong to the TGF-β (transforming growth factor beta) superfamily.24,25 They play an important role in cell differentiation, proliferation and apoptosis. The loss of BMPR2 function or reduced expression can lead to the development of PAH.24,25
The promoter region of the BMPR2 gene has a conserved coding sequence which is of special interest in gene regulation. These conserved coding sequences are associated with transcription factor binding sites and other cis-acting regulatory elements. The aim of this study was to analyze the methylation pattern of the CpG island located in the last 2000pb of the promoter region of the BMPR2 gene, specifically in the region of this island which contains the binding sequence with the greatest number of transcription factors, in DNA from the peripheral blood of IPAH and APAH patients, in order to study the effect of changes on disease penetrance.
Patients and MethodsRecruitment and Clinical Evaluation of PatientsStudy patients were recruited from hospitals in Galicia, the Hospital Valdecilla in Santander, and from the Spanish Association of Pulmonary Hypertension. Informed consent was obtained in all cases, and the study was conducted in accordance with ethical principles for medical research in humans, set down in the Declaration of Helsinki (http://www.wma.net) and published by the World Medical Association. The local Ethics Committee (the Autonomous Research Ethics Committee of Galicia) approved the study.
Patients were included consecutively, both in the hospitals and in the Spanish Association of Pulmonary Hypertension, if they met the inclusion criteria and agreed to participate in the study. Patients were diagnosed and classified on the basis of their clinical history, and according to the results of hemodynamic testing. The diagnosis of PAH was based on right heart catheterization showing mPAP≥25mmHg and pulmonary artery wedge pressure≤15mmHg with no specific treatment. In all cases the standard treatment protocol recommended by the European Respiratory Society/European Society of Cardiology was followed.26 Clinical data were collected, including functional class (FC), 6-minute walk test (6MWT), lung function, and clinical laboratory tests. Hemodynamic variables included mPAP, systolic pulmonary artery pressure, pulmonary artery wedge pressure, cardiac index, pulmonary vascular resistance, and vasoreactivity testing. Patients were stable at the time of catheterization.
Samples from 50 healthy individuals with no apparent respiratory disease selected from the general population were used to determine variations in methylation. These samples were collected for the study in the University Hospital Complex of Vigo (Spain).
Detection and Analysis of CpG Islands in the BMPR2 GeneMethyl Primer Express® Version 1.0 software was used to detect CpG islands in the promoter region of the BMPR2 gene, using the ENSEMBL reference sequence ENST00000374580. After the selected DNA sequence is introduced, the program detects all possible CpG islands and offers specific primer designs based on the target sequences selected. Methyl Primer Express® is used to design primers for methylation specific-PCR (MSP) amplification, one of the most widely used techniques in methylation mapping.
The Genomatix MatInspector program was used to analyze the selected regions to determine the existence of regulatory transcription elements and binding sites.
Methylation AnalysisDNA samples were modified with sodium bisulfite using the EZ DNA Methylation-Direct™ Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer's instructions. The starting amount of DNA recommended for optimal results is 200–500ng. In each batch of bisulfite-treated DNA samples from the blood of patients and controls, we included a negative control (untreated DNA) and a positive control treated with methyltransferase CpG (MSssI) (New England Biolabs, Ipswich, MA, USA). Both the positive control and the negative control were from the same healthy individual. For the positive control, we mixed 34.5μL of sterile H2O, 4μL of Mg2+-free buffer, 4μL of SAM 1/10, 1.8μL of M. SsssI and 6μL of DNA, which was incubated in a water bath at 37°C for 16h. After modification with bisulfite, the total DNA of the samples was quantified using the NanoDrop 2000c Spectrophotometer (Thermo Scientific, Waltham, MA, USA).
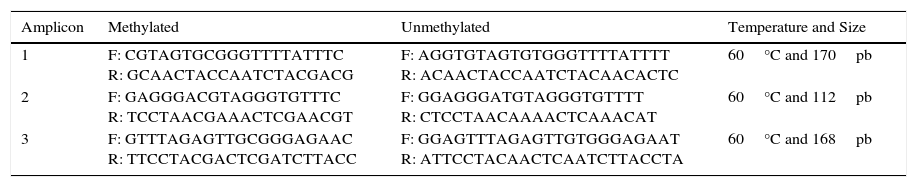
For methylation-specific PCR (MSP), 1–4μL of bisulfite-modified DNA was used. Fragments were amplified using taq TaKaRa Ex Taq Hot Start Version (TAKARA BIO Inc, Otsu, Shiga, Japan), according to the instructions of the manufacturer. The final volume of the PCR reaction was adjusted with sterile H2O to 25μL. Two PCR mixtures were prepared, one with the methylated primers and the other with the unmethylated primers (Table 1). PCR conditions were as follows: 5min at 95°C, 38 cycles of 30s at 95°C, 30s at 60°C and 30s at 72°C, terminating with a final extension at 72°C for 7min.
Primers Used for MSP Amplification of the Promoter Region of the BMPR2 Gene.
| Amplicon | Methylated | Unmethylated | Temperature and Size |
|---|---|---|---|
| 1 | F: CGTAGTGCGGGTTTTATTTC R: GCAACTACCAATCTACGACG | F: AGGTGTAGTGTGGGTTTTATTTT R: ACAACTACCAATCTACAACACTC | 60°C and 170pb |
| 2 | F: GAGGGACGTAGGGTGTTTC R: TCCTAACGAAACTCGAACGT | F: GGAGGGATGTAGGGTGTTTT R: CTCCTAACAAAACTCAAACAT | 60°C and 112pb |
| 3 | F: GTTTAGAGTTGCGGGAGAAC R: TTCCTACGACTCGATCTTACC | F: GGAGTTTAGAGTTGTGGGAGAAT R: ATTCCTACAACTCAATCTTACCTA | 60°C and 168pb |
F: forward; R: reverse.
After MSP, the total PCR volume was loaded to a 3% agarose gel with ethidium bromide at a concentration of 10μg/mL, to check the correct amplification of the PCR product DNA and the methylation status. DNA was visualized and a digital image of the gel was obtained using QuantityOne version 4.6.1 software.
H1299 Cell CulturesAs positive controls for expression, we used H1299 non-small cell lung cancer cells cultured in Dulbecco's Modified Eagle's Medium, Gibco (San Diego, CA, USA), supplemented with 10% fetal bovine serum+penicillin (100U/mL) and streptomycin (100mg/mL). After 48h in culture, when confluency was >90%, cells were detached from the culture bottles using Gibco tripsin (San Diego, CA, USA), and DNA was extracted from the culture using the FlexiGene DNA Kit (Qiagen, Germany), according to the instructions of the manufacturer. All experiments were performed in duplicate in three different assays to ensure reliability and reproducibility.
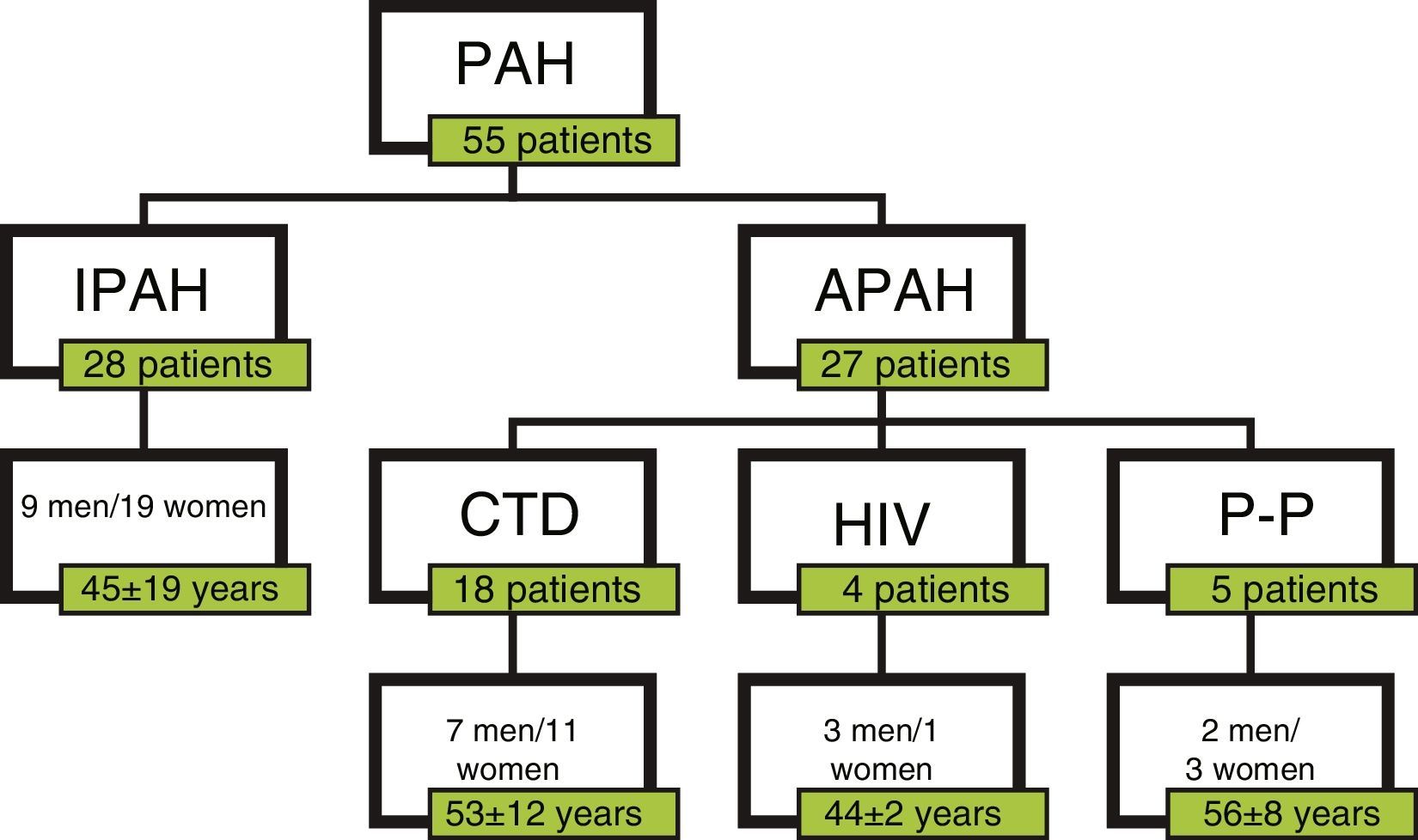
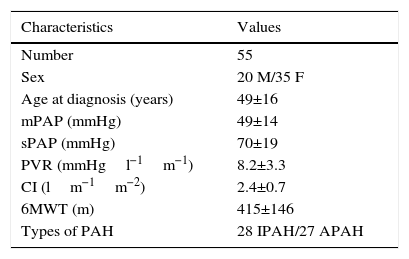
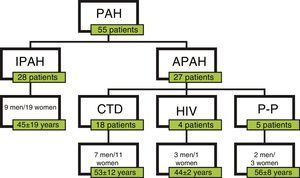
ResultsPatientsA total of 55 patients were included, 28 with a diagnosis of IPAH and 27 with APAH. At the time of diagnosis, 7 patients had FC I; 19, FC II; 25, FC III; and 4, FC IV (Fig. 1). In total, 34 patients were considered good responders. Good response after 6 months of treatment was defined as improvement by at least one FC grade, reduced propeptide of brain natriuretic peptide (proBNP) and an increase of at least 15% in the distance walked on the 6MWT. Patient characteristics are shown in Table 2.
Clinical and Hemodynamic Characteristics of Patients.
| Characteristics | Values |
|---|---|
| Number | 55 |
| Sex | 20 M/35 F |
| Age at diagnosis (years) | 49±16 |
| mPAP (mmHg) | 49±14 |
| sPAP (mmHg) | 70±19 |
| PVR (mmHgl−1m−1) | 8.2±3.3 |
| CI (lm−1m−2) | 2.4±0.7 |
| 6MWT (m) | 415±146 |
| Types of PAH | 28 IPAH/27 APAH |
Values are expressed as mean±standard deviation.
6MWT, 6-min walk test; CI, cardiac index; F, female; M, male; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; sPAP, systolic pulmonary arterial pressure.
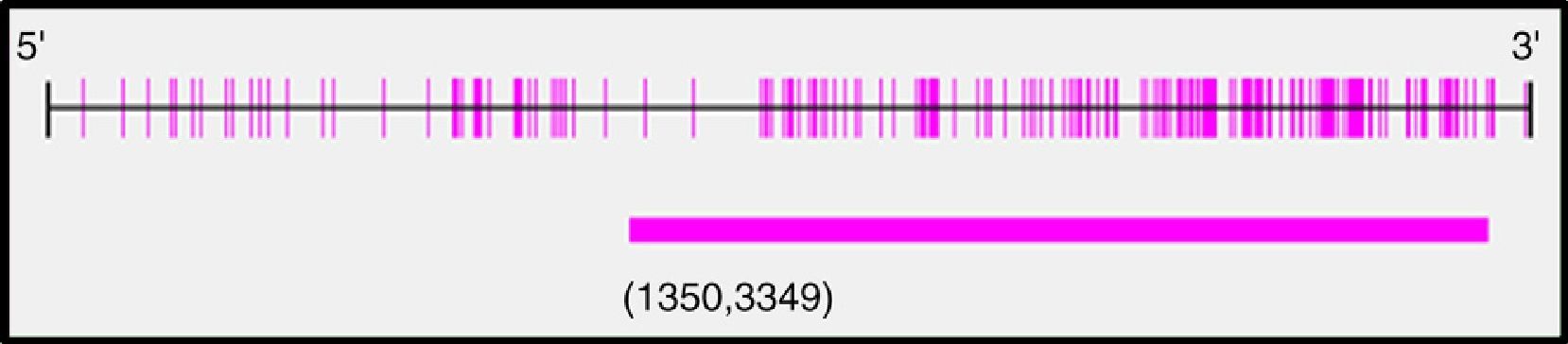
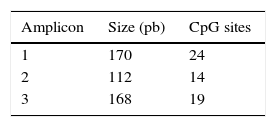
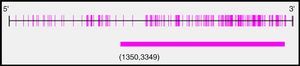
After analysis of the last 2000pb of the promoter region of the BMPR2 gene using the Methyl Primer Express® Version 1.0, a long CpG island of 1999pb, suitable for methylation, was identified (Fig. 2). The amplicons selected for amplification from the complete fragment each had different lengths and numbers of CpG sites; amplicon 1 was the longest and amplicon 3 had the most CpG sites (Table 3).
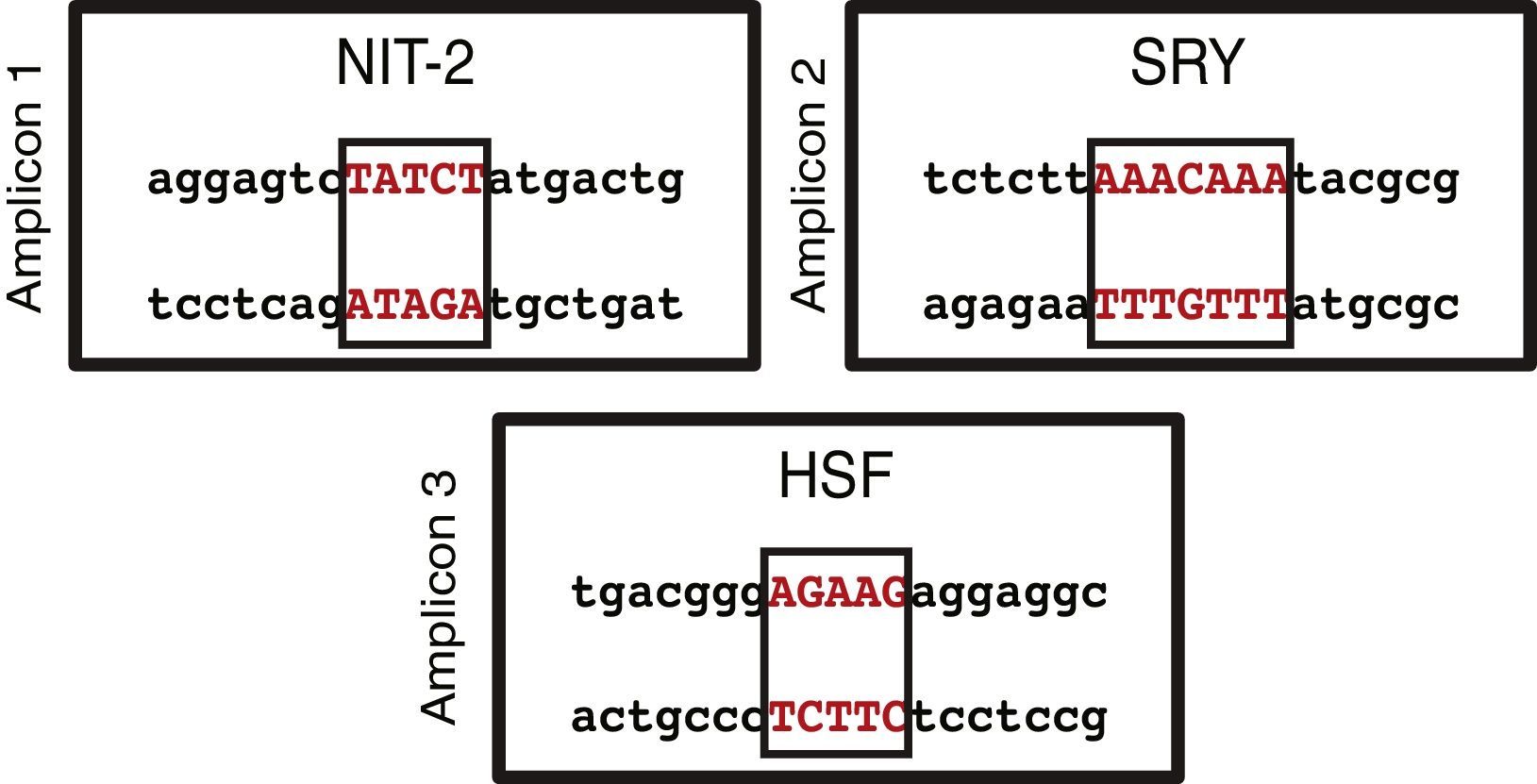
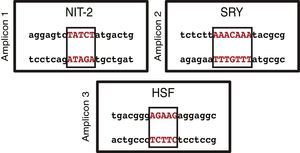
On the basis of the sequence entered in the MatInspector software and the structural information stored in its database, transcription factor binding sites were identified in the three amplicons analyzed. Using a reliability of ≥90%, each of the amplicons revealed several binding sites. When we were more restrictive and raised the reliability to 100%, we only found 1 transcription factor binding site in each of the amplicons (Fig. 3). NIT-2 (global-acting regulatory protein) was localized on amplicon 1, sex-determining region Y on amplicon 2, and heat shock factor on amplicon 3. However, none of the three transcription factor binding sites detected was located among the localized CpG sites.
Methylation Analysis of the BMPR2 Gene Promoter RegionFor bisulfite modification, a concentration of genomic DNA of 450ng was used for each sample. The mean concentration of bisulfite-treated DNA obtained was 26.68±9.39ng/μL. The lowest concentration obtained in one of the patients was 10ng/μL and the highest was 71ng/μL.
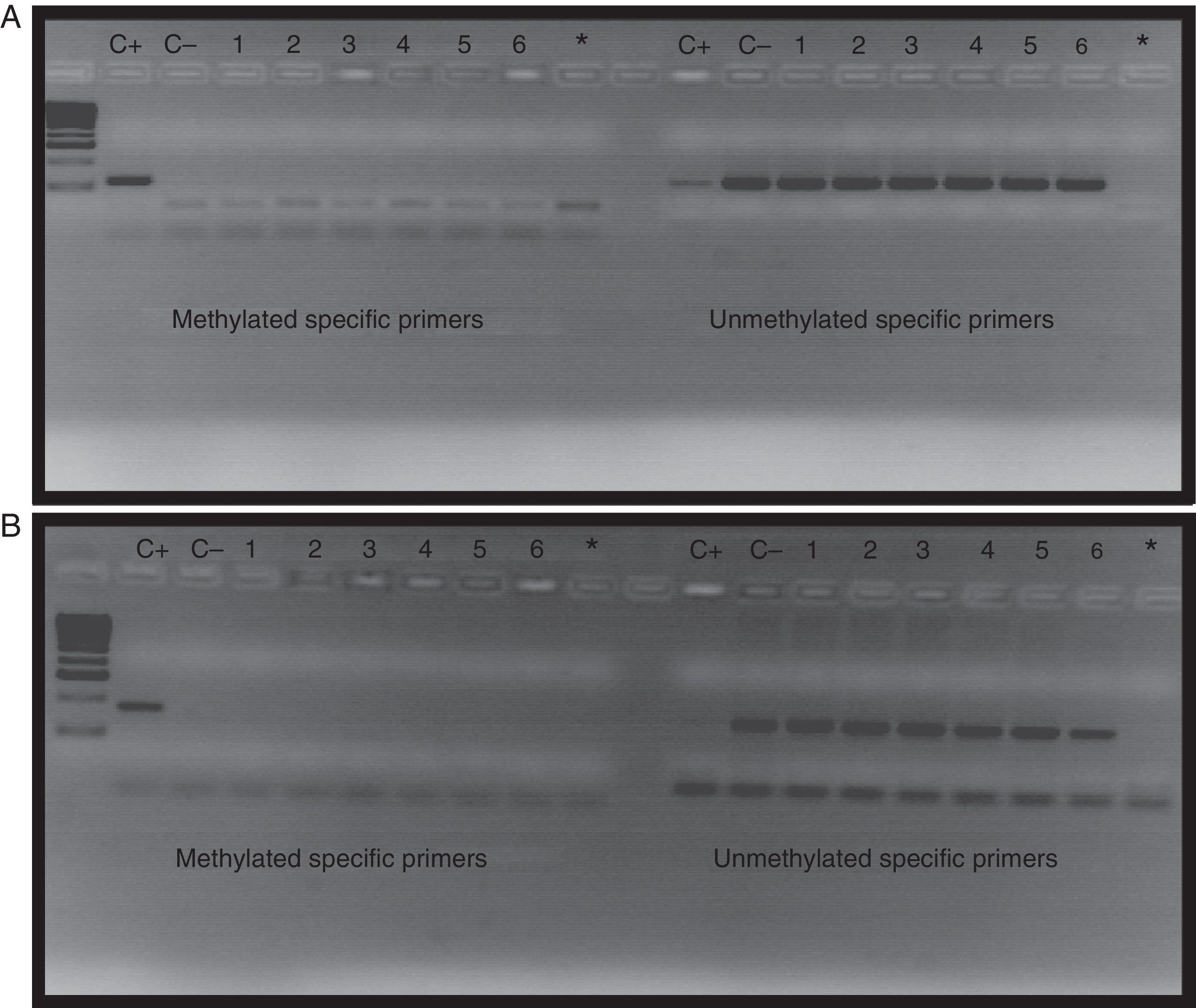
The BMPR2 gene promoter region studied was negative for methylation in both the 55 patients with PAH and the 50 controls. No patient showed signs of methylation after epigenetic mapping. Fig. 4 shows an example of the methylation pattern of amplicon 3, seen in 6 patients randomly selected from the 55 included in the study.
Amplification of the BMPR2 gene promoter regions in patients with PAH (A) and controls (B). Wells 1–6 correspond to the result of methylation-specific PCR amplification and the non-methylation-specific PCR of patients; wells C+ correspond to methylated controls, and wells C− to unmethylated controls and Wells * to targets with H2O.
H1299 cells, which express BMPR2 endogenously, were used for extracting DNA and for determining whether the promoter region of the study gene was methylated in a specific tissue where it is expressed. We did not find any indication of methylation in this region in any of the amplicons studied. Fig. 5 shows an example of the methylation pattern of amplicon 2, present in the 6 H1299 cell culture replicas.
Amplification of the BMPR2 gene promoter regions in H1299 Cells. Wells 1–6 correspond to the result of methylation-specific PCR amplification and the non-methylation-specific PCR of replicas of H1299 cell cultures; wells C+ correspond to methylated controls, and wells C− to unmethylated controls and wells * to targets with H2O.
Epigenetics is the branch of genetics which studies and characterizes changes in phenotypes or gene expression that are not involved in DNA sequencing per se.18–22 In our patient series, no positive results were found for CpG site methylation in the promoter region we analyzed, despite this being a region which contains a large number of transcription factor binding sites and a large CpG island, susceptible to methylation. The same results were obtained for our control cases, suggesting that methylation of the BMPR2 gene promoter region is not associated with PAH appearance, development or penetrance.
The BMPR2 gene is expressed in smooth muscle cells of the pulmonary arteries, and human genome methylation profiles are tissue-specific. However, the high cost of detecting global methylation levels in the whole genome means that researchers have begun to use specific profiles in substitute tissues, such as blood.27,28 In this study, we used DNA from peripheral blood leukocytes, a very common practice in epigenetic mapping, when access to the target tissue is problematic.27 Some authors, such as Li et al., have reported that peripheral blood can be used as a tissue for determining methylation patterns in different genes related with different types of cancer, producing significant differences between patients and controls, although not in all cases.28 The advantage of using peripheral blood is that it is easy to obtain high-quality DNA, and the use of this method has become widespread. It has been suggested that immunological processes associated with inflammation in different diseases can alter the methylation pattern of leukocyte subpopulations,28 so greater insight into the origin and nature of leukocyte DNA is needed to confirm its suitability as a substitute for specific tissue DNA for determining methylation patterns in a gene with hard-to-access expression. Hamid et al.29,30 have described BMPR2 gene expression in human B lymphocytes, confirming the validity of using peripheral blood in our analysis. Other genes which can produce changes in patient phenotypes, such as ACVRL1 and endoglin are also expressed, like BMPR2, in the smooth muscle cells of the pulmonary arteries. Accordingly, we investigated BMPR2 methylation patterns in smooth muscle cells of the pulmonary arteries which did express this gene, and the results were negative. This finding suggests that in healthy patients the promoter region of this gene might be demethylated, and if it is methylated, it may alter gene expression or even silence it. To confirm the methylation pattern in patients with PAH, it would be necessary to repeat the study using tissue-specific DNA from smooth muscle cells of the pulmonary arteries, or even to use animal models in which the affected tissue is easily accessed.
The MatInspector database search for transcription factor binding sites identified three potential binding sites: NIT-2 (global-acting regulatory protein), sex-determining region Y, and heat shock factor.31–33 These transcription factors may be altered if there is a methylated CpG site in their sequence. This would be very interesting, as these transcription factors have important regulatory effects on the different cell and viral promoters and also can act as transcriptional activators. Accordingly, if the promoter region of this gene is methylated and if the sequence of any of these binding sites should change, preventing the correct assembly of transcription factors, this may have serious physiological effects triggered by the dysregulation of the BMPR2 gene.
One of the main characteristics of epigenetic changes is that they can be inherited. Perhaps, then, the ideal situation would be to analyze these changes in patients with FPAH. Moreover, other epigenetic mechanisms, such as histone modification, chromatin remodeling or interfering RNA may be involved in PAH; it would be interesting to analyze these factors, since they can activate or disactivate the genes controlling cell growth, proliferation and apoptosis. In contrast, demethylation of CpG sites has been associated with the overexpression of oncogenes in carcinogenic cells. Demethylation can cause dysregulation of the transcriptional activity of the hypomethylated gene. However, in our study, we did not find any evidence that the BMPR2 gene was unmethylated, since no differences were found between patients and controls.
The method used in this study for determining methylation status (methylated or unmethylated) involved visualization in agarose gel, which is an observer-dependent technique. This could be improved with the use of a quantification strategy, using quantitative methylation in methylation-specific qPCR. Using this method, it may be possible to determine and estimate the percentage of methylation in patients for the studied gene, instead of simply observing the presence or absence of methylation.34,35
One of the potential limitations of this study is the relatively small number of patients analyzed, although the fact that we did not find any case seems quite conclusive.
In conclusion, in view of the complex and multifactorial nature of PAH, it seems likely that epigenetic events might play a role in the development or progression of this disease. Some authors have suggested that epigenetic changes may occur in the promoter region of the BMPR2 gene in PAH patients. This region is probably the most suitable for this type of analysis, due to its numerous transcription factor binding sites which, if they are modified by any epigenetic change, may alter gene function. However, in our study, we did not find any differences in the methylation pattern between patients and healthy subjects.
FundingThis study was funded by the SOGAPAR project IN-202-05, Actelion Pharmaceuticals project CO-0118-2012, and the Xunta de Galicia INBIOMED project 2009-063.
Authorship/co-workersGP was responsible for study design, genetic analysis, data analysis, correlations, and manuscript writing. AB was responsible for collecting samples and clinical data, analyzing the data, and manuscript writing. DV was responsible for study design and coordination, data analysis, and manuscript writing. All the authors read and approved the final manuscript.
Conflict of interestsAdolfo Baloira has received research grants from Actelion and has provided consultancy services to Actelion, GSK, Pfizer, Lilly, Bayer and Ferrer.
The other authors state that they have no conflict of interests.
We would like to express our thanks to the patients who gave their consent to participate in this study. We would also like to thank the physicians who assisted in the recruitment of patients (Carlos Vilariño Pombo, José Manuel Cifrián and Olalla Añón) and, finally, we would like to thank the Spanish Association of Pulmonary Hypertension for their collaboration with their BIOCAPS Support Program (Biomedical Aptitudes Support Program) FP7-REGPOT316265.
Please cite this article as: Pousada G, Baloira A, Valverde D. Análisis de la metilación de la región promotora del gen BMPR2 en pacientes con hipertensión arterial pulmonar. Arch Bronconeumol. 2016;52:293–298.