Patients with Duchenne's muscular dystrophy (DMD) have a high incidence of constipation, and the chronic use of laxatives is an important component of their treatment. It has been postulated that the underlying cause of constipation in these patients is the functional deterioration of the smooth muscles of the gastrointestinal tract, which can cause gastric dilation, slowing of gastric and ileal emptying, and even intestinal pseudo-occlusion. Pathological and functional smooth muscle abnormalities appear to be derived from dystrophin deficiency, the locus-encoded protein associated with DMD.1 Gastric or intestinal dilation may be aggravated specifically in patients with hypercapnic respiratory failure, due to the ingestion of air during non-invasive ventilation (NIV).2 It is well known that treatment with laxatives can lead to the development of metabolic acidosis,3,4 an electrolyte disturbance that could have respiratory consequences in patients with chronic hypoventilation.
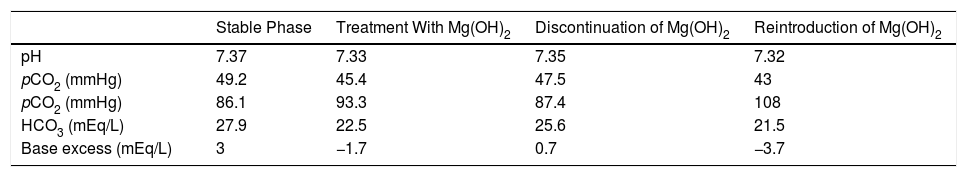
We report the case of a 37-year-old DMD patient with chronic hypercapnic respiratory failure, who required NIV 12h a day. A stable phase lung function study showed: FEV1/FVC 94%, FVC 26%, FEV1 30%, MIP 12%, MEP 8%, peak cough flow 149L/min, pH 7.37, pO2 86.1mmHg, pCO2 49.2mmHg, HCO3 27.9mEq/L. Routine outpatient monitoring revealed iron deficiency anemia, so oral iron was added to his usual treatment (betahistine, dihydrochloride, and paracetamol). The patient's chronic constipation worsened, so magnesium hydroxide [Mg(OH)2] was started as a laxative. Successive follow-ups detected a worsening of the acid-base balance with the appearance of metabolic acidosis (see Table 1) and an increase in ventilatory support needs of up to 18–20h a day. The patient's targeted clinical history showed no focus of infection. Blood gases also showed a decrease in pCO2 and bicarbonate (HCO3). Correct renal function and the presence of a normal anion gap (sodium 139mmol/L, chlorine 102mmol/L) were confirmed. Given the suspicion of metabolic acidosis caused by the use of magnesium hydroxide, this compound was replaced with bisacodyl and lactitol. Subsequent blood gas monitoring showed the correction of acidosis, presenting results similar to the baseline status. Two years later, the patient reinitiated magnesium hydroxide treatment for persistent constipation, resulting in a new episode of metabolic acidosis (see Table 1).
Course of Blood Gases.
| Stable Phase | Treatment With Mg(OH)2 | Discontinuation of Mg(OH)2 | Reintroduction of Mg(OH)2 | |
|---|---|---|---|---|
| pH | 7.37 | 7.33 | 7.35 | 7.32 |
| pCO2 (mmHg) | 49.2 | 45.4 | 47.5 | 43 |
| pCO2 (mmHg) | 86.1 | 93.3 | 87.4 | 108 |
| HCO3 (mEq/L) | 27.9 | 22.5 | 25.6 | 21.5 |
| Base excess (mEq/L) | 3 | −1.7 | 0.7 | −3.7 |
HCO3: bicarbonate; Mg(OH)2: magnesium hydroxide; pCO2: partial pressure of carbon dioxide; pO2: partial pressure of oxygen.
The chronification or worsening of metabolic acidosis can have several consequences, including cardiac contractility changes with decreased cardiac output, increased incidence of arrhythmias, arterial and venous vasodilation, increased pulmonary vascular resistance, metabolic demands, insulin resistance, anaerobiosis due to reduced adenosine-triphosphate synthesis (ATP), hyperkalemia, and alterations in the level of consciousness.5,6
Specifically, magnesium hydroxide is an osmotic laxative that can disturb the acid–base balance by the loss of bicarbonate, and the higher the loss, the greater the degree of acidosis.3,7,8 The initial response to bicarbonate loss is decreased pCO2 due to the stimulation of ventilation, producing hyperventilation to normalize pH. On average, the loss of 1mEq/L of HCO3 is compensated by a 1.2mmHg drop in pCO2,9 but this response cannot be maintained, whether metabolic acidosis becomes chronic or if the patient is unable to respond to the respiratory demand, and may result in muscle fatigue, altered ventilation mechanics and increased NIV needs. Based on this mechanism, the use of acidifying drugs as part of the treatment of residual hypercapnia in patients receiving home mechanical ventilation has been proposed.10 However, in patients with neuromuscular diseases, the compensatory hyperventilation mechanism could lead, as in this case, to an increase in the work of breathing.
It is therefore important to be cautious when using osmotic laxatives in patients with neuromuscular pathology and chronic respiratory failure receiving treatment with home mechanical ventilation, as the consequences could be particularly severe.
Please cite this article as: Peñacoba P, Antón A, Güell MR. Acidosis metabólica por laxantes en paciente con distrofia muscular de Duchenne y ventilación mecánica no invasiva. Arch Bronconeumol. 2020;56:530–531.