Pleural infections have high morbidity and mortality, and their incidence in all age groups is growing worldwide. Not all infectious effusions are parapneumonic and, in such cases, the organisms found in the pleural space are not the same as those observed in lung parenchyma infections. The diagnostic difficulty lies in knowing whether an infectious effusion will evolve into a complicated effusion/empyema, as the diagnostic methods used for this purpose provide poor results.
The mainstays of treatment are to establish an early diagnosis and to commence an antibiotic regimen and chest drain as soon as possible. This should preferably be carried out with fine tubes, due to certain morphological, bacteriological and biochemical characteristics of the pleural fluid. Fluid analysis, particularly pH, is the most reliable method for assessing evolution. In a subgroup of patients, fibrinolytics may help to improve recovery, and their combination with DNase has been found to obtain better results. If medical treatment fails and surgery is required, video-assisted thoracoscopic surgery (VATS) is, at least, comparable to decortication by thoracotomy, so should only undertaken if previous techniques have failed.
Further clinical trials are needed to analyze factors that could affect the results obtained, in order to define new evidence-based diagnostic and therapeutic strategies that provide more effective, standardized management of this disease.
Las infecciones pleurales presentan una elevada morbimortalidad, y su incidencia está aumentando en todos los países del mundo y en todos los grupos de edad. No todos los derrames infecciosos son paraneumónicos, y en esos casos, los organismos que se encuentran en el espacio pleural no son los mismos que se observan en las infecciones del parénquima pulmonar. La dificultad diagnóstica radica en saber si un derrame infeccioso evolucionará hacia un derrame complicado/empiema, ya que los métodos diagnósticos utilizados con este fin ofrecen pobres resultados.
Los pilares del tratamiento son establecer un diagnóstico precoz e instaurar, lo antes posible, una pauta antibiótica y un drenaje torácico. Este se llevará a cabo, preferiblemente con tubos de pequeño calibre, ante la presencia de determinadas características morfológicas, bacteriológicas y bioquímicas del líquido pleural. El análisis del líquido es el método más fiable para valorar su evolución, sobre todo la determinación del pH. En un subgrupo de pacientes los fibrinolíticos pueden contribuir a mejorar la recuperación, y su combinación con deoxirribonucleasa se relaciona con la obtención de mejores resultados. Si fracasa el tratamiento médico y es necesaria la cirugía, la rentabilidad de la cirugía toracoscópica videoasistida es, al menos, comparable a la decorticación por toracotomía, por lo que esta solamente se realizará si han fallado las técnicas anteriores.
Son necesarios más ensayos clínicos que analicen factores que puedan influir sobre los resultados obtenidos para conformar nuevas estrategias diagnósticas y terapéuticas basadas en la evidencia, que proporcionen un manejo más efectivo y estandarizado de esta enfermedad.
Parapneumonic pleural effusion (PPE) refers to a pleural effusion (PE) associated with bacterial pneumonia, a pulmonary abscess, or infected bronchiectasis.1 When not accompanied by parenchymal disease, it is known as a pleural infection (PI) or complicated PE (CPE). Although most PPEs can be resolved with antibiotic treatment alone, a subgroup of patients can present severe complications, such as: PPE refractory to antibiotic treatment and chest drainage (CD), requiring surgical drainage (33%)2; pleural fibrosis (14%); prolonged hospital stay (mean 12–15 days and >1 month in 25% of cases)2,4,5; and high mortality rates (10–20%).2,4,6 In order to avoid these, a firm diagnosis must be reached as soon as possible, and close clinical monitoring established.
EpidemiologyOf the one million patients hospitalized annually in the United States for pneumonia, around 60,000 will present empyema, and a further 25,000 will develop it for other reasons.7 Despite clinical advances, the incidence of PI, irrespective of its stage of development, is increasing worldwide5,8 across all age groups.9–11 In the United States, the rate of hospitalization for empyema doubled from 1996 to 2008 (from 3.04 to 5.98/100,000 population), across all age groups.12
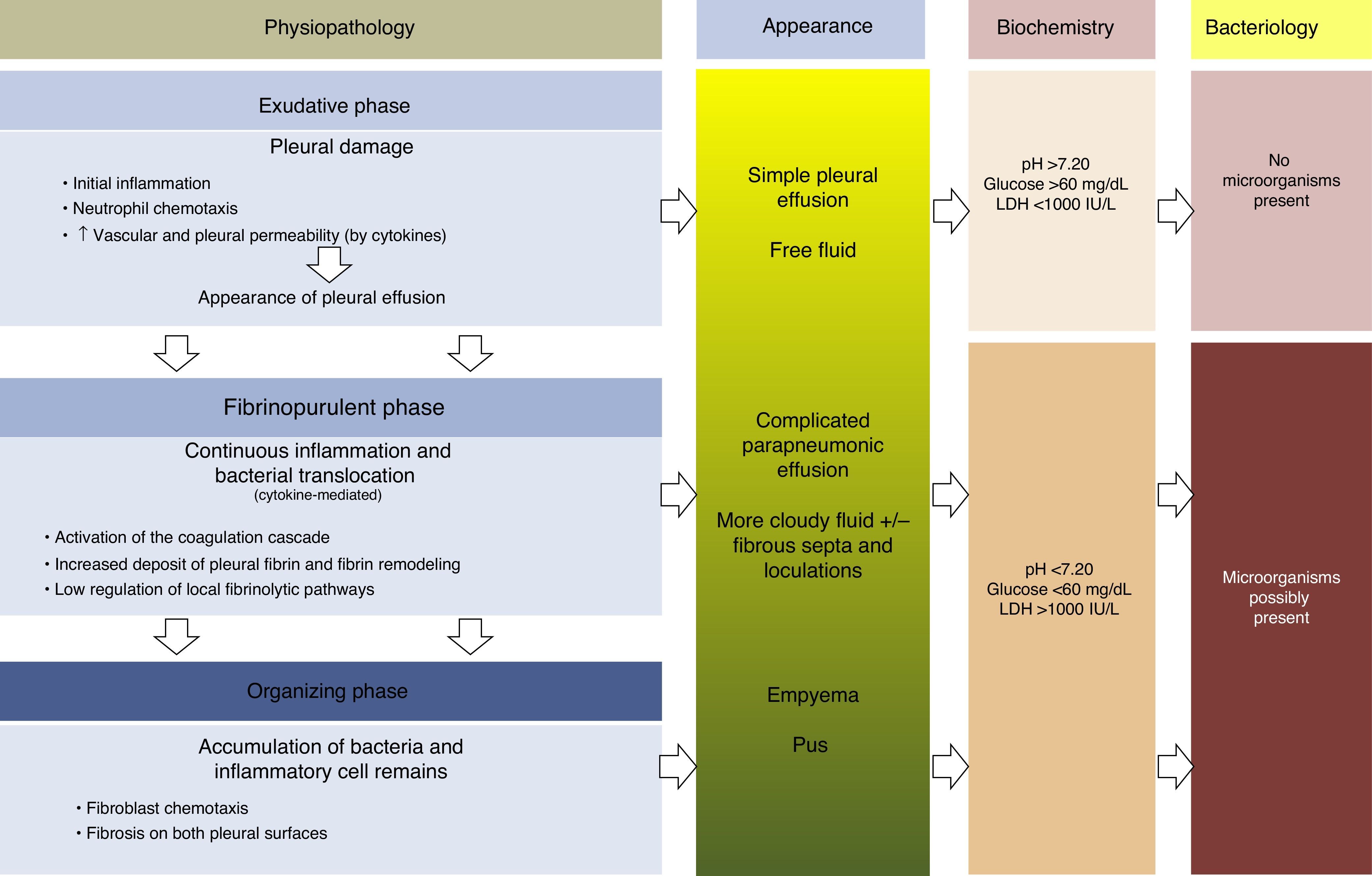
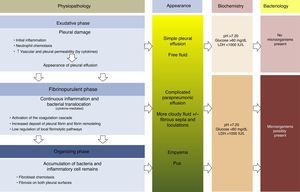
PhysiopathologyThere are 3 phases in the evolution of PPE. In the first (exudative) phase, pleural fluid (PF) forms as a consequence of localized inflammation and activation of the immune system. The activated neutrophils can cause an endothelial lesion, which will increase capillary permeability and lead to onset of PE.13,14 The pleural damage can lead to commencement of the second phase (fibrinopurulent), with the activation of pro-inflammatory and pro-fibrinotic components, and initiation of the coagulation cascade. The local inflammatory response is thus amplified by the presence of various cytokines, which stimulate chemotaxis of neutrophils and fibroblasts.14 Furthermore, membrane permeability can allow bacteria to enter the pleural space. In the latter stages of this phase, PF resembling empyema may be observed due to the presence of cellular degradation products and bacterial remains. During this period, there is also a decrease in fibrinolysis, thereby increasing fibrin formation in the pleural space. This process can progress to the final (organizing) phase, in which a layer of fibrosis is formed on both pleural surfaces due to the increase in fibroblastic infiltration. As a result, these cases can be extremely difficult to manage without resorting to surgery15,16 (Fig. 1). The appearance, analysis and culture of PE can vary in each of the evolutionary phases of PPE. The physiopathological changes that take place in the pleural space mean that, from a clinical point of view, PPE can be classified as: uncomplicated PPE (UPPE), which is resolved with antibiotic treatment; complicated PPE (CPPE), which will require CD or surgery for resolution; and empyema, i.e. the presence of pus in the pleural space, which must always be drained.
Physiopathology, appearance, biochemical parameters and microbiology of parapneumonic pleural effusion.
Diagnosing PPE is not complex in patients presenting with classic symptoms, which are similar to pneumonia.17 A poor response to pneumonia therapy could suggest the presence of a PPE or empyema as a complication of the disease.18 It is sometimes difficult to suspect a PI, as the symptoms are atypical, and there is no evidence of pneumonia on chest radiograph.19 Blood cultures are only positive in 12% of cases, PF culture is negative in more than 40% of samples2 and, occasionally, the microorganisms responsible are very rare and can only be identified by molecular microbiology.20 Although the criteria for defining CPPEs are well established,17 there are no clinical or radiological data to determine which patients will go on to develop CPPE/empyema. As these are easily identifiable by their appearance, the difficulty lies in detecting (as early as possible) which patients with non-purulent PPE might progress to CPPE. Various diagnostic methods have been used for this purpose, but results have so far been disappointing.
Pneumonia severity scales and scoresPneumonia severity scales18,21 have been used to predict 30-day mortality following admission, but not to assess the likelihood of developing CPPE/empyema. Chalmers et al.22 showed that these scales and generic sepsis scoring systems (APACHE II, SEWS and SIRS) cannot predict the development of CPPE/empyema. However, multivariate logistic regression has identified that: albumin<30g/l, Na<130mmol/l, platelet count>400,000, C-reactive protein (CRP)>100mg/l and a history of alcohol abuse and intravenous drug use are independent risk factors for CPPE/empyema.22
Rahman et al.23 developed a validated clinical risk score (RAPID) to identify patients with PI and high risk of dying, to enable clinicians to implement the best management strategy. The score includes urea levels, age, presence of purulent PF, source of infection (community or hospital), and dietary factors (albumin). Patients can then be divided into low, medium and high-risk groups according to the score. The odds ratio for mortality at 3 months for the medium- and high-risk groups, using the low-risk group as a reference, is 24.4 and 192.4, respectively. Using this system, patients with PI at presentation can be stratified according to risk.
Imaging testsRadiographs, chest ultrasound (CU) and chest computed tomography (CT) can provide information on the size, extent and nature of the PE. PF volumes of >200–250cm3 are usually seen on the chest radiograph, and CPPE may be suspected if opacification of the pleural space does not change with the gravitational effects of the PF.
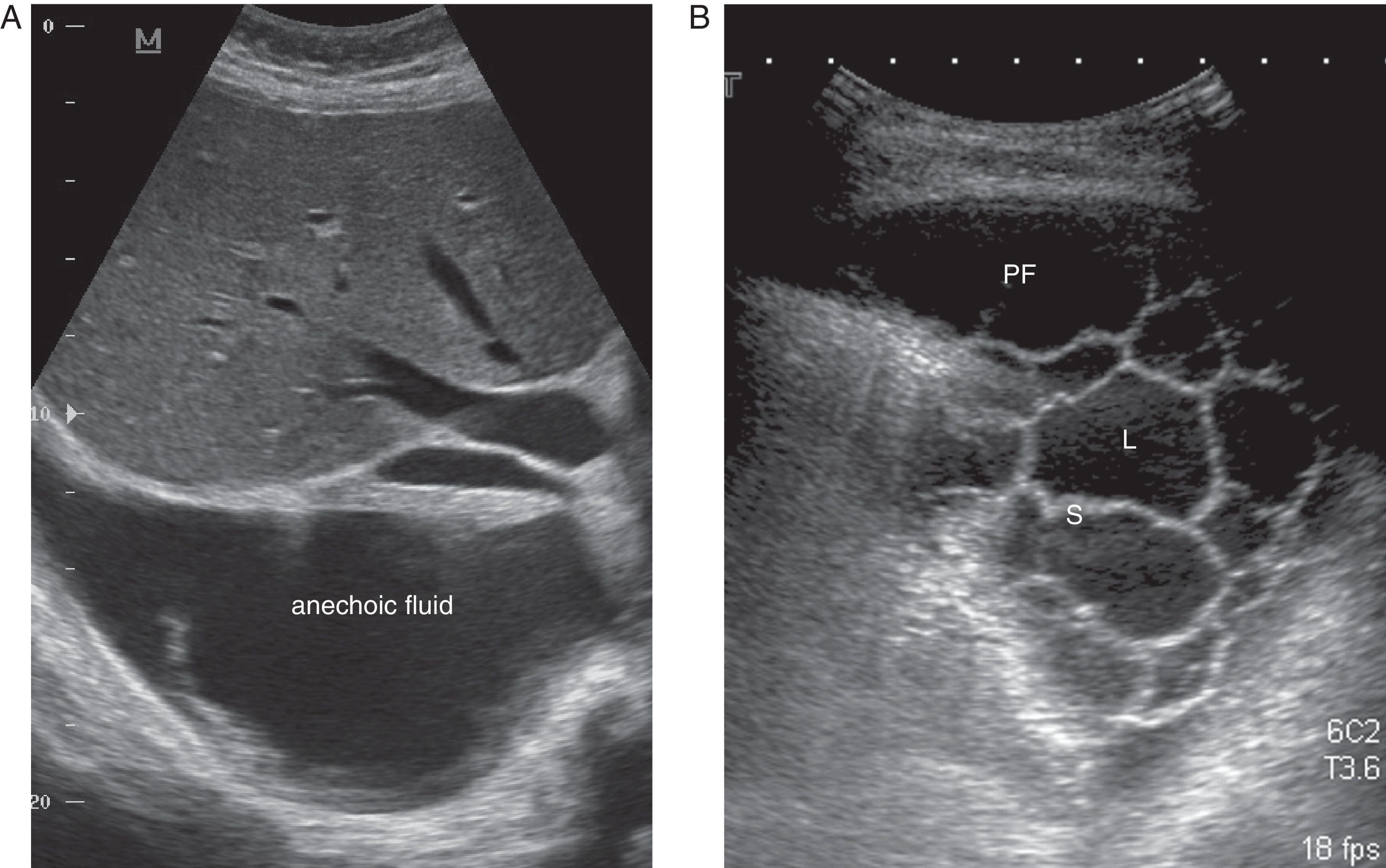
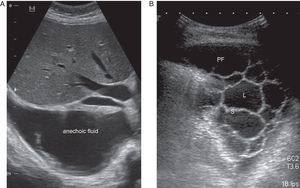
CU is a key technique in the management of PE, particularly PPE, and pulmonologists must be competent in its use. It is more sensitive than radiography for detecting minor PE, establishes the echogenicity of the PF, precisely locates the loculated fluid, estimates the volume and depth of the PE, differentiates between PF and underlying consolidation or atelectasia, improves the yield of thoracocentesis, and reduces the risk of complications compared to physical examination plus radiography.24 An ultrasound finding of septa suggests CPPE (Fig. 2), and hyperechogenicity is associated with pus in the pleural cavity.
CT provides the clearest image of the pleura, and can be used to reconstruct images, determine loculations, and reveal lesions in the underlying lung. It also differentiates between a peripheral pulmonary abscess and a loculated PI, though the split pleura sign.25
CU, being radiation-free and easily performed at the bedside in critically ill patients, should be the technique of choice. Factors that may affect image quality, such as obesity or subcutaneous emphysema, and suspicion of malignant PE or pleural infection with comorbidity (esophageal rupture, bronchopleural fistula, etc.), in which other anatomical abnormalities must be identified, may make it necessary to perform a CT scan.
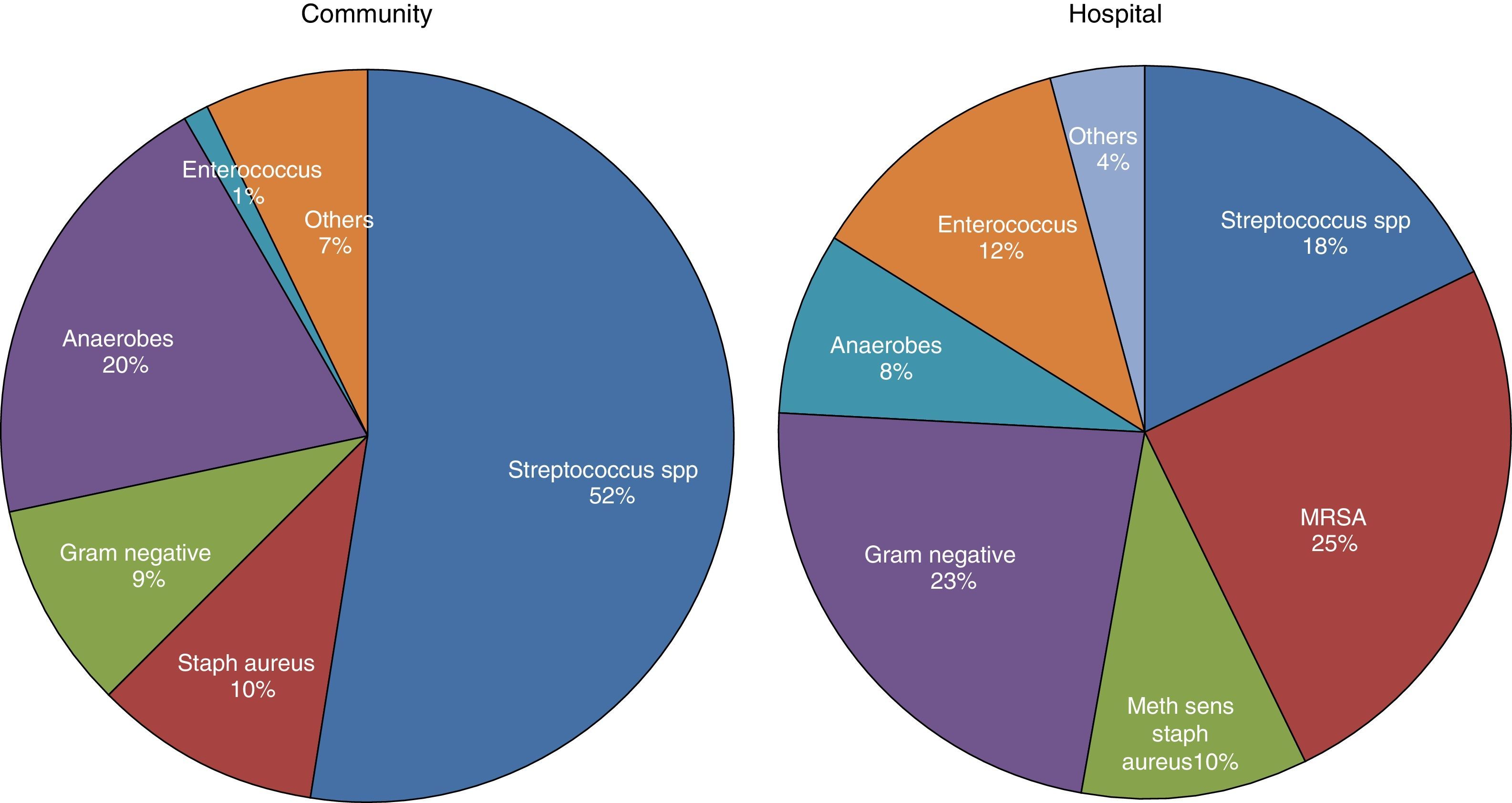
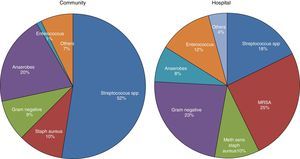
MicrobiologyA positive PF culture is diagnostic of PI, but this only occurs in 58% of cases. This proportion increases to 74% if molecular microbiology is used.20 PI is thought to occur as a result of migration of the bacteria present in the lung parenchyma, so such infections are usually called CPPEs, on the assumption that the bacterial etiology of all PIs is the same as that of pneumonia. However, in a review of 14 studies with a total of 1383 patients with empyema, only 70% were due to pneumonia and the rest were due to other causes.26 Therefore, basing the choice of antibiotic for treating PI on this assumption is not the best strategy, as there are major bacteriological differences between PI and pneumonia.20 These differences are probably due to significant differences in acidity and oxygenation between the infected pleural space and the ventilated lung, thus predisposing it to invasion by certain organisms over others.27 There are also differences (as regards the types of microorganisms) between community- and hospital-acquired PIs20 (Fig. 3).
Microbiology of community- and hospital-acquired pleural infection.20 MRSA, Methicillin-resistant Staphylococcus aureus.
The heptavalent pneumococcal vaccine could play a major role in the evolution of the microbiology of PI, since it has been observed that the predominant serotypes that cause PI are those not covered by the vaccine.28 This seems to correlate with a significant increase in the incidence of empyema,29 which suggests an increase in the virulence of the new microorganisms.
Pleural fluid analysisThe combination of clinical symptoms, physical examination, blood tests and chest radiography usually confirms the presence of pneumonia with PPE. PF analysis is the most reliable method for diagnosing the condition, and for trying to prevent progression to CPPE.30 Thoracocentesis is therefore recommended in all patients with suspected infectious PE if the pleural fluid thickness in the chest radiograph obtained in lateral decubitus is >10mm,19 although the likelihood of a PPE<2cm in depth on the CT scan progressing to CPPE is small.31
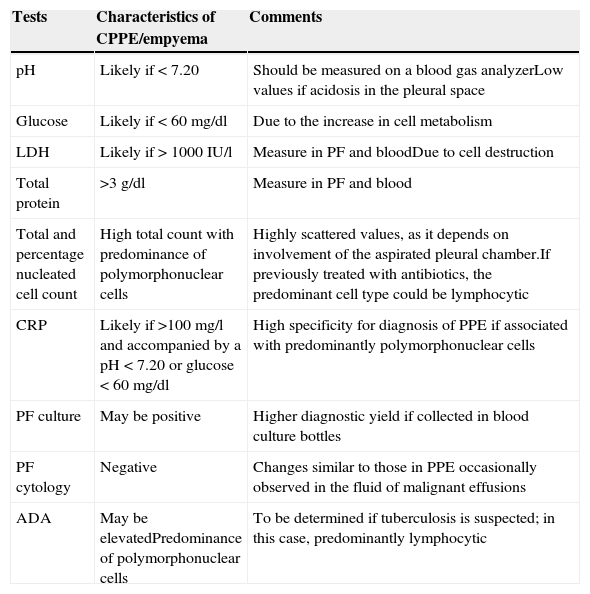
PF analysis can vary in each of the evolutionary phases of PPE. The tests to be performed when PPE is suspected are shown in Table 1. A UPPE is a polymorphonuclear (PMN) predominant exudate with a serous appearance, normal biochemical findings, and negative culture. If the patient has previously received antibiotics, the nucleated cells will be predominantly lymphocytic.32 Biochemistry tests in PF, however, are most useful as a tool for early detection CPPEs/empyema. While empyema always requires drainage, the decision to drain a non-purulent PPE is based on the morphological (>1/2 hemithorax, loculations and thickening of the parietal pleura), bacteriological (culture) and biochemical characteristics (pH<7.20) of the PE.19,33 Lactate dehydrogenase (LDH)>1000IU/l and glucose<60mg/dl do not improve the diagnostic yield, and will only be used if the pH cannot be determined.19 Factors such as air or traces of heparin or local anesthetic in the sample syringe can alter the pH results.34 Furthermore, PIs caused by Proteus spp. can secrete enzymes that alkalize the PF.35 Some patients with an initial pleural pH>7.20 may also require CD and even surgery. This is due to the heterogeneity of the biochemical characteristics in the loculated PE, since the degree of involvement of the aspirated chamber can significantly affect disease severity indices, as determined by pH. Therefore, although pleural pH is highly specific (91.8%)36 in predicting the need for CD, it is less sensitive in determining mortality or the eventual need for surgery.4,19 While leading scientific societies accept this parameter for draining a PE,19,33 a retrospective analysis has shown that this test is not highly specific in establishing the need for CD in non-purulent PE, i.e. a group of patients with UPPE are considered as CPPE, which results in the needless placement of chest drains.37 The total nucleated cell count and percentage of PMN leucocytes are usually higher in CPPE/empyemas.38
Pleural fluid testing when an infectious pleural effusion is suspected.
| Tests | Characteristics of CPPE/empyema | Comments |
|---|---|---|
| pH | Likely if < 7.20 | Should be measured on a blood gas analyzerLow values if acidosis in the pleural space |
| Glucose | Likely if < 60mg/dl | Due to the increase in cell metabolism |
| LDH | Likely if > 1000IU/l | Measure in PF and bloodDue to cell destruction |
| Total protein | >3g/dl | Measure in PF and blood |
| Total and percentage nucleated cell count | High total count with predominance of polymorphonuclear cells | Highly scattered values, as it depends on involvement of the aspirated pleural chamber.If previously treated with antibiotics, the predominant cell type could be lymphocytic |
| CRP | Likely if >100mg/l and accompanied by a pH<7.20 or glucose<60mg/dl | High specificity for diagnosis of PPE if associated with predominantly polymorphonuclear cells |
| PF culture | May be positive | Higher diagnostic yield if collected in blood culture bottles |
| PF cytology | Negative | Changes similar to those in PPE occasionally observed in the fluid of malignant effusions |
| ADA | May be elevatedPredominance of polymorphonuclear cells | To be determined if tuberculosis is suspected; in this case, predominantly lymphocytic |
ADA, adenosine deaminase; CPPE, complicated parapneumonic pleural effusion; CRP, C-reactive protein; LDH, lactate dehydrogenase; PF, pleural fluid; PPE, parapneumonic pleural effusion.
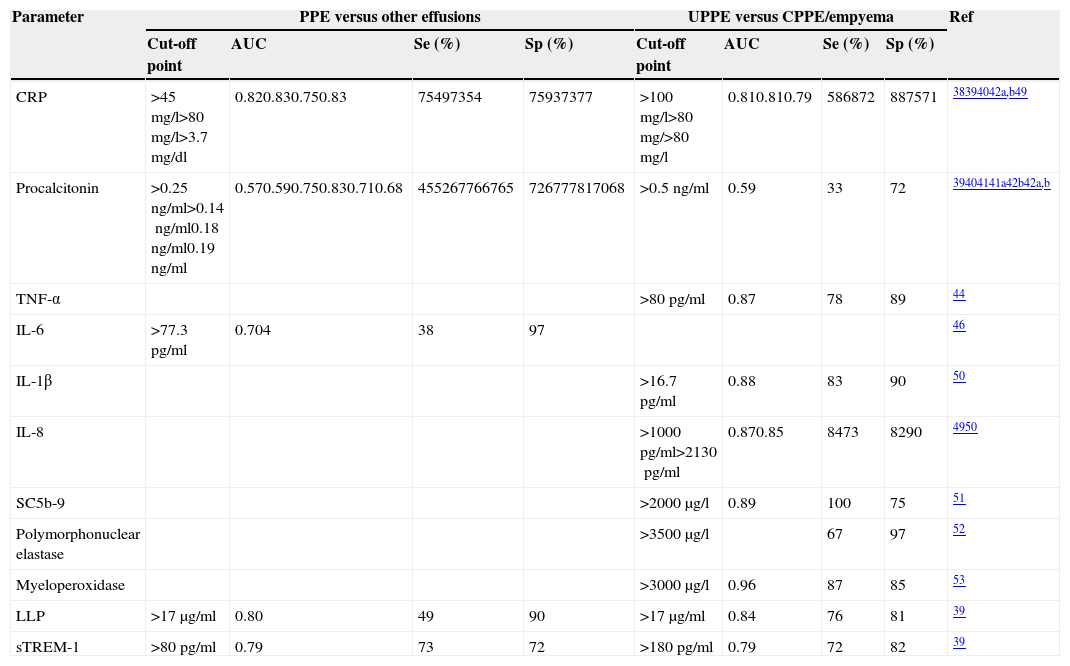
CRP has been used to differentiate both PPE from non-parapneumonic exudates,38–41 and UPPE from CPPE38,39 (Table 2). It should be noted that the specificity of this test increases (96%38 and 92%40) if high CRP values are combined with a predominance of PMNs in the PF. Moreover, if a CRP>100mg/l is accompanied by a pH<7.20 or glucose<60mg/dl, the specificity for the diagnosis of CPPE is 97%.38
Diagnostic yield of alternative biomarkers in pleural fluid for the diagnosis of parapneumonic pleural effusions.
| Parameter | PPE versus other effusions | UPPE versus CPPE/empyema | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cut-off point | AUC | Se (%) | Sp (%) | Cut-off point | AUC | Se (%) | Sp (%) | ||
| CRP | >45mg/l>80mg/l>3.7mg/dl | 0.820.830.750.83 | 75497354 | 75937377 | >100mg/l>80mg/>80mg/l | 0.810.810.79 | 586872 | 887571 | 38394042a,b49 |
| Procalcitonin | >0.25ng/ml>0.14ng/ml0.18ng/ml0.19ng/ml | 0.570.590.750.830.710.68 | 455267766765 | 726777817068 | >0.5ng/ml | 0.59 | 33 | 72 | 39404141a42b42a,b |
| TNF-α | >80pg/ml | 0.87 | 78 | 89 | 44 | ||||
| IL-6 | >77.3pg/ml | 0.704 | 38 | 97 | 46 | ||||
| IL-1β | >16.7pg/ml | 0.88 | 83 | 90 | 50 | ||||
| IL-8 | >1000pg/ml>2130pg/ml | 0.870.85 | 8473 | 8290 | 4950 | ||||
| SC5b-9 | >2000μg/l | 0.89 | 100 | 75 | 51 | ||||
| Polymorphonuclear elastase | >3500μg/l | 67 | 97 | 52 | |||||
| Myeloperoxidase | >3000μg/l | 0.96 | 87 | 85 | 53 | ||||
| LLP | >17μg/ml | 0.80 | 49 | 90 | >17μg/ml | 0.84 | 76 | 81 | 39 |
| sTREM-1 | >80pg/ml | 0.79 | 73 | 72 | >180pg/ml | 0.79 | 72 | 82 | 39 |
AUC, area under the curve; CPPE, complicated parapneumonic pleural effusion; CRP, C-reactive protein; IL, interleukin; LBP, lipopolysaccharide-binding proteins; PPE, parapneumonic pleural effusion; Ref, reference; SC5b-9, soluble form of C5b-9; Se, sensitivity; Sp, specificity; sTREM-1, soluble triggering receptor expressed on myeloid cells-1; TNF-α, tumor necrosis factor alpha; UPPE, uncomplicated parapneumonic pleural effusion.
Although procalcitonin (PCT) values are high in bacterial infections, the advantage of determining this peptide in PF and its capacity to differentiate CPPEs from UPPEs remains unclear.39–42 Nevertheless, a recent study confirmed that PCT is a specific biomarker for infection, and that PCT levels are not affected by non-infectious inflammation.43 Furthermore, PCT differentiates PPE from non-infectious PPE better than CRP, although it does not predict the need for surgery or risk of death.43
The diagnostic yield of other parameters such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-1β, IL-8, complement products (soluble form of C5b-9), neutrophil-derived enzymes (polymorphonuclear elastase and myeloperoxidase), lipopolysaccharide binding protein (LBP), and soluble receptor activator expressed in myeloid cells (sTREM-1), are summarized in Table 2.39,44–53 There is skepticism among investigators about the likelihood of a single parameter (especially without taking into account clinical, radiological or microbiological data) being capable of diagnosing PI with sufficient certainty to change current clinical practice.54
TreatmentTreatment is based on controlling infection with appropriate antibiotics, draining the CPPE/empyema, assessing the use of fibrinolytics and existing surgical options, ensuring good nutrition, and administering anti-thrombotic prophylaxis.55
AntibioticsThe use of antibiotics should be based on local microbiological recommendations and on the specific risks of each patient. Treatment should be initiated empirically and promptly, and adjusted to the community or nosocomial origin of the infection, culture results, and activity of the antibiotic in the PF.56 In community-acquired PIs, the recommended combination is amoxicillin-clavulanic acid as monotherapy, or a third-generation cephalosporin and clindamycin/metronidazole. If the patient is allergic to penicillin, a quinolone can be combined with clindamycin/metronidazole. In nosocomial PIs, in which methicillin-resistant Staphylococcus aureus is very common,20 the empirical regimen should cover this and anaerobic microorganisms (e.g. vancomycin/linezolid, anti-Pseudomonas penicillins, carbapenem or third-generation cephalosporins combined with metronidazole). Macrolides are not generally used, since although pneumonia caused by Legionella or Mycoplasma pneumoniae can present with PE, it is usually self-limiting and rarely causes empyema.57 The use of aminoglycosides is not recommended, due to their poor penetration in the pleural space, nor is there evidence to recommend the intra-pleural use of antibiotics.16
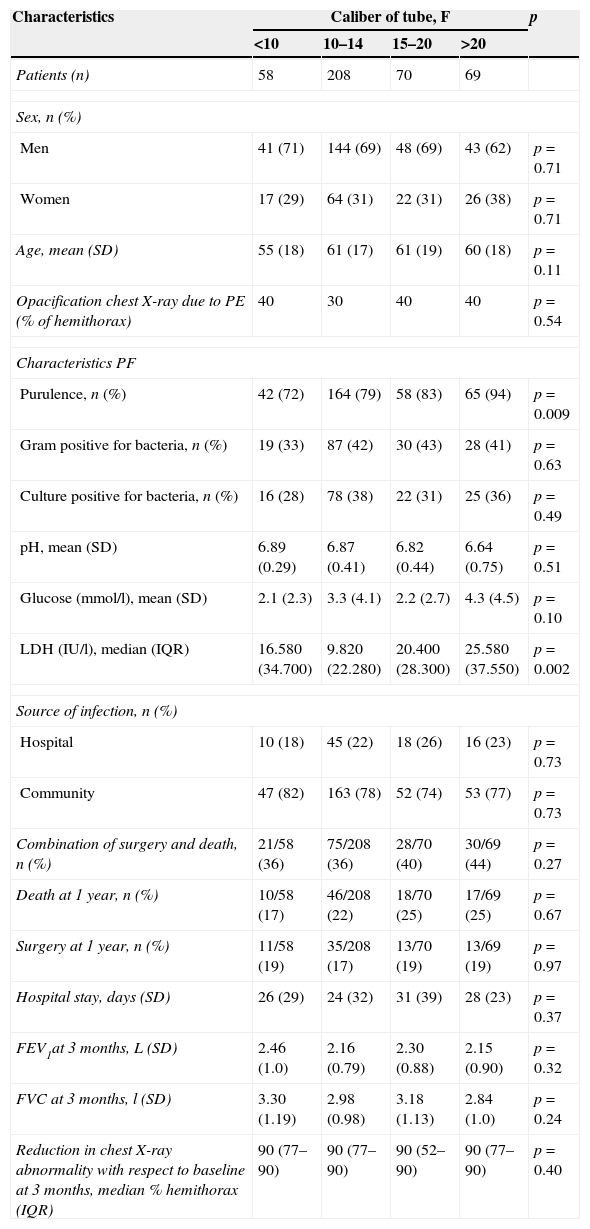
Chest drainageAlthough there is no consensus on the caliber of the drainage tube, British Thoracic Society19 guidelines and other studies58 suggest that small bore tubes (10–14F) are suitable for most CPPEs, as they are easier to place, are less traumatic and painful for the patient, and are as effective as larger bore tubes (Table 3). Insertion of a chest catheter should always be image-guided, generally using CU. It is indicated in patients with cloudy or purulent PF, positive PF staining or culture, loculation or PE greater than half the hemithorax, pH<7.20, or thickening of the parietal pleura.
Patient characteristics according to the size of the chest-tube caliber and the effect of the caliber on primary and secondary clinical outcomes.
| Characteristics | Caliber of tube, F | p | |||
|---|---|---|---|---|---|
| <10 | 10–14 | 15–20 | >20 | ||
| Patients (n) | 58 | 208 | 70 | 69 | |
| Sex, n (%) | |||||
| Men | 41 (71) | 144 (69) | 48 (69) | 43 (62) | p=0.71 |
| Women | 17 (29) | 64 (31) | 22 (31) | 26 (38) | p=0.71 |
| Age, mean (SD) | 55 (18) | 61 (17) | 61 (19) | 60 (18) | p=0.11 |
| Opacification chest X-ray due to PE (% of hemithorax) | 40 | 30 | 40 | 40 | p=0.54 |
| Characteristics PF | |||||
| Purulence, n (%) | 42 (72) | 164 (79) | 58 (83) | 65 (94) | p=0.009 |
| Gram positive for bacteria, n (%) | 19 (33) | 87 (42) | 30 (43) | 28 (41) | p=0.63 |
| Culture positive for bacteria, n (%) | 16 (28) | 78 (38) | 22 (31) | 25 (36) | p=0.49 |
| pH, mean (SD) | 6.89 (0.29) | 6.87 (0.41) | 6.82 (0.44) | 6.64 (0.75) | p=0.51 |
| Glucose (mmol/l), mean (SD) | 2.1 (2.3) | 3.3 (4.1) | 2.2 (2.7) | 4.3 (4.5) | p=0.10 |
| LDH (IU/l), median (IQR) | 16.580 (34.700) | 9.820 (22.280) | 20.400 (28.300) | 25.580 (37.550) | p=0.002 |
| Source of infection, n (%) | |||||
| Hospital | 10 (18) | 45 (22) | 18 (26) | 16 (23) | p=0.73 |
| Community | 47 (82) | 163 (78) | 52 (74) | 53 (77) | p=0.73 |
| Combination of surgery and death, n (%) | 21/58 (36) | 75/208 (36) | 28/70 (40) | 30/69 (44) | p=0.27 |
| Death at 1 year, n (%) | 10/58 (17) | 46/208 (22) | 18/70 (25) | 17/69 (25) | p=0.67 |
| Surgery at 1 year, n (%) | 11/58 (19) | 35/208 (17) | 13/70 (19) | 13/69 (19) | p=0.97 |
| Hospital stay, days (SD) | 26 (29) | 24 (32) | 31 (39) | 28 (23) | p=0.37 |
| FEV1at 3 months, L (SD) | 2.46 (1.0) | 2.16 (0.79) | 2.30 (0.88) | 2.15 (0.90) | p=0.32 |
| FVC at 3 months, l (SD) | 3.30 (1.19) | 2.98 (0.98) | 3.18 (1.13) | 2.84 (1.0) | p=0.24 |
| Reduction in chest X-ray abnormality with respect to baseline at 3 months, median % hemithorax (IQR) | 90 (77–90) | 90 (77–90) | 90 (52–90) | 90 (77–90) | p=0.40 |
F, French; IQR, interquartile range; PE, pleural effusion; PF, pleural fluid; SD, standard deviation.
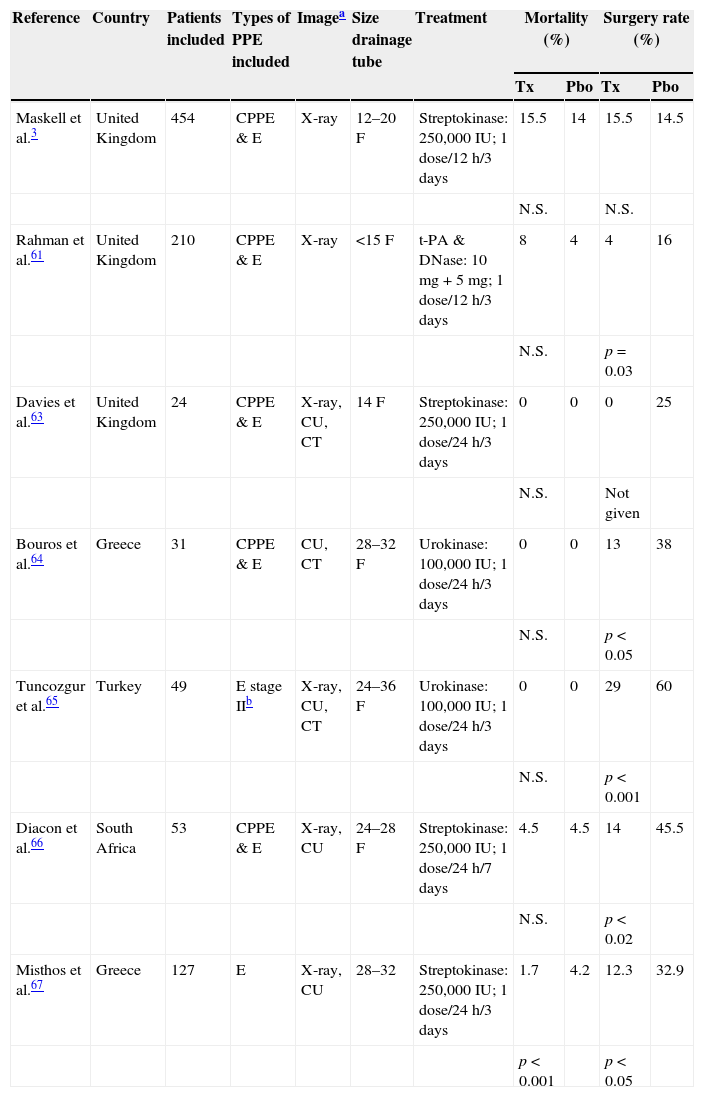
There are no indications in current guidelines for the routine use of intrapleural fibrinolytics in patients with PI,19 and their systematic use in the management of CPPE is controversial.59 A Cochrane review confirmed that intrapleural fibrinolytics are beneficial in both reducing treatment failure and the need for surgery in loculated PEs or empyema, although they do not affect mortality.60 In patients who received alteplase (a tissue plasminogen activator) and deoxyribonuclease (DNase), pleural opacity on radiography, need for surgery at 3 months, and mean hospital stay were significantly improved compared to placebo.61 A meta-analysis that included this study (and others using streptokinase and urokinase) showed heterogeneous results, but suggested that treatment with fibrinolytics reduces the need for surgery, but not the risk of mortality (Table 4).3,61–67 Current guidelines recommend the prompt use of fibrinolytics when there are loculations in the pleural cavity (resistant to CD) and in empyema. There is insufficient evidence to recommend one drug over another, and no consensus on the recommended dose.19,55 Guidelines also recommend closing the CD tube for a few hours after fibrinolytic administration.19,61
Intrapleural fibrinolytic treatment. Characteristics of the placebo-controlled trials.
| Reference | Country | Patients included | Types of PPE included | Imagea | Size drainage tube | Treatment | Mortality (%) | Surgery rate (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tx | Pbo | Tx | Pbo | |||||||
| Maskell et al.3 | United Kingdom | 454 | CPPE & E | X-ray | 12–20F | Streptokinase: 250,000IU; 1 dose/12h/3 days | 15.5 | 14 | 15.5 | 14.5 |
| N.S. | N.S. | |||||||||
| Rahman et al.61 | United Kingdom | 210 | CPPE & E | X-ray | <15F | t-PA & DNase: 10mg+5mg; 1 dose/12h/3 days | 8 | 4 | 4 | 16 |
| N.S. | p=0.03 | |||||||||
| Davies et al.63 | United Kingdom | 24 | CPPE & E | X-ray, CU, CT | 14F | Streptokinase: 250,000IU; 1 dose/24h/3 days | 0 | 0 | 0 | 25 |
| N.S. | Not given | |||||||||
| Bouros et al.64 | Greece | 31 | CPPE & E | CU, CT | 28–32F | Urokinase: 100,000IU; 1 dose/24h/3 days | 0 | 0 | 13 | 38 |
| N.S. | p<0.05 | |||||||||
| Tuncozgur et al.65 | Turkey | 49 | E stage IIb | X-ray, CU, CT | 24–36F | Urokinase: 100,000IU; 1 dose/24h/3 days | 0 | 0 | 29 | 60 |
| N.S. | p<0.001 | |||||||||
| Diacon et al.66 | South Africa | 53 | CPPE & E | X-ray, CU | 24–28F | Streptokinase: 250,000IU; 1 dose/24h/7 days | 4.5 | 4.5 | 14 | 45.5 |
| N.S. | p<0.02 | |||||||||
| Misthos et al.67 | Greece | 127 | E | X-ray, CU | 28–32 | Streptokinase: 250,000IU; 1 dose/24h/3 days | 1.7 | 4.2 | 12.3 | 32.9 |
| p<0.001 | p<0.05 | |||||||||
CPPE, complicated parapneumonic pleural effusion; CT, computed tomography; CU, chest ultrasound; E, empyema; F, French; N.S., not significant; Pbo, placebo group; PPE, parapneumonic pleural effusion; Tx, treatment group.
Surgery (video-assisted thoracoscopic surgery [VATS] drainage; thoracotomy with pleural decortication, or other type of open surgical drainage) must be considered when medical treatment (antibiotics, CD and fibrinolytics) fails after 5–7 days,16,19,55 or when organized empyema is established and there is evidence of significant pleural fibrosis. The early finding of purulent PF or loculations is not predictive of surgery.2 In addition to pleural debridement, VATS facilitates decortication in advanced or chronic empyema. In the latter, the outcomes are poorer, as the highest success rates are obtained when the procedure is performed at an early stage. Nevertheless, the overall success rate of this technique exceeds 85%.68 Several studies have compared conservative treatment (antibiotics and CD with/without fibrinolytics) with VATS as first line treatment in both children and adults. Early surgery has not shown any advantages in mortality or severe morbidity in any trials.69 Moreover, surgery is not free from complications, particularly post-surgical pleural intercostal neuralgia. Decortication by thoracotomy in empyema is being increasingly replaced by VATS, in light of evidence that the latter is at least comparable, or even better,70 and is usually reserved for cases in which less invasive procedures have failed.
NutritionPoor nutrition is an adverse determinant in pleural empyema outcome, although this factor is not generally taken into account. PIs associated with hypoalbuminemia have a worse outcome,16,19,55 and patients should be given adequate nutritional support, including complementary enteral feeding, as soon as diagnosis is confirmed.
Anti-thrombotic prophylaxisAll patients hospitalized for pneumonia and/or PI should receive prophylaxis with low molecular weight heparins, if these are not contraindicated.16,19,55
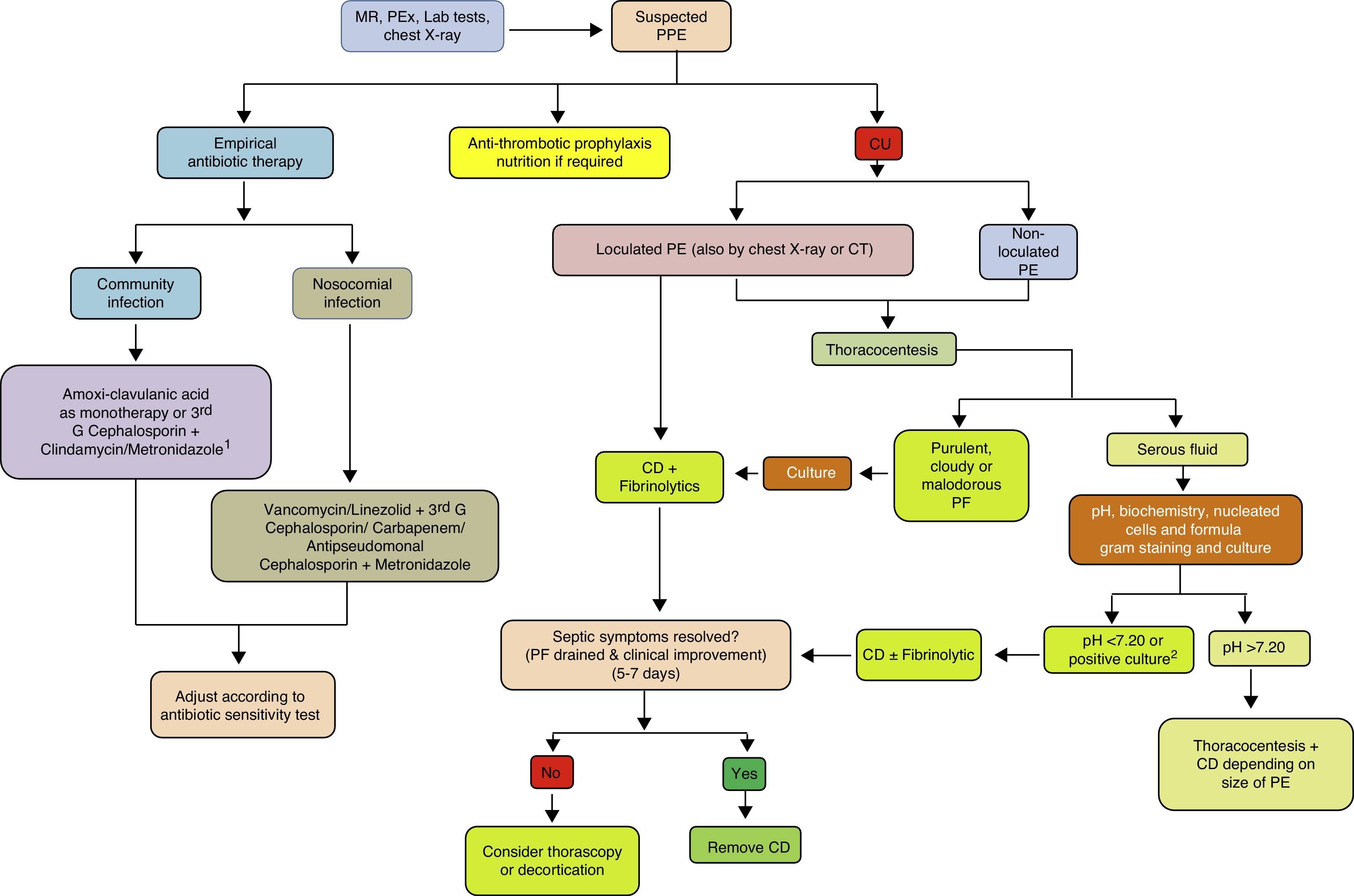
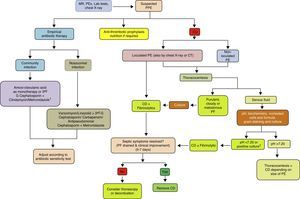
ConclusionsThe incidence of PPE is increasing worldwide, across all age groups. Although related with pneumonia, there is little correlation between organisms found in the pleural space and those observed in lung parenchyma infections, suggesting that primary PI is more common than previously thought. Current diagnostic methods focus on being able to predict whether a PPE will progress to a CPPE/empyema. Diagnosis and early strategies such as antibiotic regimens and CT continue to form the basis of treatment. In a sub-group of patients, fibrinolytics help to improve recovery, and have been associated with better outcome when combined with DNase. If surgery is necessary, there is increasing evidence that VATS is comparable to, if not better than, decortication by thoracotomy, and so should be reserved for cases in which less invasive procedures have failed (Fig. 4). Further clinical trials are needed to define evidence-based diagnostic and therapeutic strategies that provide more effective, standardized management of this disease.
Treatment algorithm for parapneumonic pleural effusion. CD, chest drainage; CT, computed tomography; CU, chest ultrasound; G, generation; MR, medical record; PE, pleural effusion; PEx physical examination; PF, pleural fluid; PPE, parapneumonic pleural effusion. 1 If allergic to penicillin: quinolone+metronidazole. 2 If the pH cannot be determined, use LDH>1000IU/l or glucose<60mg/dl.
Ferreiro L. and Valdés L. are the authors and editors and also contributed in conception, design and final approval of the manuscript. San José ME is the co-author and editor. He helped in final approval of the manuscript.
Conflict of interestThe authors declare that they have no conflict of interests.
Please cite this article as: Ferreiro L, San José ME, Valdés L. Manejo del derrame pleural paraneumónico en adultos. Arch Bronconeumol. 2015;51:637–646.