The cause of exudative pleural effusion cannot be determined in some patients. The long-term outcomes of patients with undiagnosed pleural effusion were analyzed.
MethodsPatients with exudative pleural effusion whose diagnostic procedures included pleural biopsy using video-assisted thoracoscopic surgery carried out between 2008 and 2012 were evaluated retrospectively. Patients diagnosed with nonspecific pleuritis were included. Fifty-three patients with available follow-up data were included in the study.
ResultsForty men and 13 women (mean age 53.9±13.9years) were included. Median follow-up time was 24months. No diagnosis was given in 27 patients (51%), and a clinical diagnosis was given in 26 patients (49%) during the follow-up period. Malignant disease (malignant mesothelioma) was diagnosed in 2 (3.7%) patients. Other diseases were parapneumonic effusion in 12, congestive heart failure in 8, and miscellaneous in 4 patients. Volume of effusion at the time of initial examination and re-accumulation of fluid after video-assisted thoracoscopic surgery were associated with malignant disease (P=.004 and .0001, respectively).
ConclusionAlthough the probability is low, some patients with exudative pleural effusion undiagnosed after pleural biopsy via video-assisted thoracoscopic surgery may have malignant disease. Patients with an initially large volume of effusion that re-accumulates after examination should be closely monitored.
En algunos pacientes no es posible establecer la causa del derrame pleural exudativo. Se han analizado los resultados del seguimiento a largo plazo de pacientes con derrame pleural no diagnosticado.
MétodosSe evaluó retrospectivamente a pacientes con derrame pleural exudativo a los que se les había realizado una biopsia pleural mediante cirugía toracoscópica videoasistida (VATS) entre 2008 y 2012 como parte de los procedimientos diagnósticos. En el estudio se incluyó a 53 pacientes con diagnóstico de pleuritis inespecífica y con datos de seguimiento disponibles.
ResultadosSe incluyó a 40 varones y 13 mujeres (promedio de edad, 53,9±13,9años). La mediana de tiempo de seguimiento fue de 24meses. Durante el seguimiento no se llegó a un diagnóstico en 27 pacientes (51%) y se alcanzó diagnóstico clínico en los 26 pacientes restantes (49%). A 2 pacientes (3,7%) se les diagnosticó neoplasia maligna (mesotelioma maligno). Otras enfermedades diagnosticadas fueron derrame paraneumónico en 12 casos, insuficiencia cardíaca congestiva en 8 casos y otras afecciones en 4 pacientes. El volumen del derrame en la exploración inicial y la reacumulación de fluido tras VATS se asociaron a neoplasia maligna (p=0,004 y 0,0001, respectivamente).
ConclusiónAunque la probabilidad es baja, los pacientes con derrame pleural exudativo y sin diagnóstico tras una biopsia pleural mediante VATS pueden tener neoplasia maligna. Es necesario controlar cuidadosamente a los pacientes con un volumen de derrame inicial alto que reaparece tras la exploración.
Pleural effusion is frequently identified in patients with pulmonary or pleural diseases, and it can also occur in association with some systemic diseases, medication use and organ dysfunction.1
The cause of exudative pleural effusion (EPE) can usually be determined through microbiological, biochemical and cytological analyses of a fluid sample, and sometimes by examination of a closed pleural biopsy. However, no underlying disease is found in ∼20% of patients with EPE.2,3 A thoracoscopic examination and evaluation of pleural biopsies may be diagnostic in over 90% of these patients.4–7 In this study, we investigated diseases that occurred during long-term follow-up in patients without a specific pleural disease diagnosis despite analyses of their pleural fluid samples and pleural biopsies obtained by video-assisted thoracoscopic surgery (VATS). We determined the likelihood of diseased pleura in this patient group. We also identified variables that indicated the existence of diseased pleura.
Patients and MethodsWe retrospectively evaluated and followed up patients with a diagnosis of EPE at our clinic between January 2008 and December 2012. Patients with lung, pleural or mediastinal tumors were excluded. Patients with pleural biopsy specimens obtained by VATS were selected to ensure that the effusion had been adequately investigated. Those with a specific histopathological diagnosis received appropriate treatment. We identified 61 patients with no specific diagnosis; histopathological findings from their pleural specimens were interpreted as acute and chronic inflammation or fibrosis of varying degrees. Of these patients, 53 with available follow-up data were included from the study. These 53 participants were examined in our clinic regularly, and pleural fluid and specimens were obtained and re-examined when necessary. Data on the prognosis of these patients during follow-up and their latest diagnoses were recorded in patient files and entered into the hospital's electronic database. This study was performed in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the Yedikule Chest Diseases and Thoracic Surgery Training and Research Hospital. Following ethics committee approval, the data obtained during the initial examination of these patients and their follow-up and imaging data were retrieved. Variables were analyzed retrospectively.
The volume of pleural effusion was categorized according to whether the fluid occupied less or more than 50% of the hemithorax, as determined from chest X-rays taken during the initial examination.
Fluid samples were obtained by diagnostic thoracentesis from all patients and analyzed. Biochemical parameters included lactate dehydrogenase (LDH), protein, albumin, pH and adenosine deaminase, and cells in fluid samples were counted and typed. Cytological examination of fluids from all patients was performed. The fluid specimens of some patients were tested for Mycobacterium tuberculosis (direct microscopy and specific culture). Closed pleural biopsy using an Abram's needle was performed in 27 patients; pathology results reported “nonspecific pleuritis.”
VATS was performed in the operating room under general anesthesia by thoracic surgeons experienced in VATS procedures. Incision points were determined according to the location of the fluid and the condition of the pleura and lungs. Parietal pleura samples were obtained from a region thought to be diseased. If no such region was evident, at least two parietal pleura samples were obtained from various regions of the pleura, each with a diameter of ∼3cm.
The mean follow-up period of patients with no specific diagnosis was 22.91 months (median 24 months; range 6–60 months).
During follow-up, a diagnosis of heart failure was given when impaired cardiac function was identified and the fluid regressed with diuretic treatment. A diagnosis of parapneumonic effusion was given when pleural inflammation was seen during VATS and clinical complaints and biochemical parameters improved after appropriate antibiotic therapy.
StatisticsThe Chi-squared or Fisher's exact test was used to compare frequencies, and Spearman's test was used to evaluate correlations. P<0.05 was considered to indicate a significant difference.
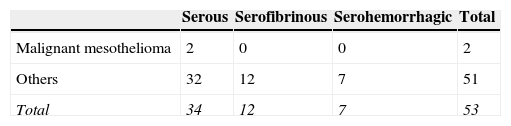
ResultsForty patients were men and 13 were women (mean age 53.9±13.9 years; range 27–77 years). Twenty-nine patients were smokers and 24 were nonsmokers. Only 16 patients complained of chest pain. The pleural fluid appearance was serous in 34 patients, serofibrinous in 12, and serohemorrhagic in 7 patients.
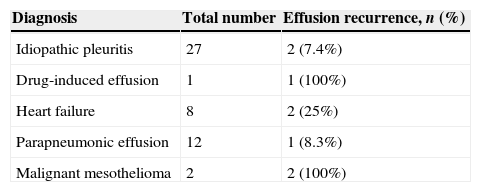
During the follow-up period, no specific diagnosis was given in 27 (51%) of the patients. Effusion did not relapse in 24 of these patients. Re-accumulation of effusion was observed in only 3 patients; however, no invasive intervention was attempted because their clinical conditions were stable and the volume of re-accumulated fluid was small. A diagnosis of “idiopathic pleuritis” was accepted in patients in whom no specific diagnosis could be made.
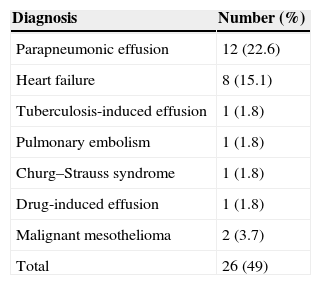
Based on symptoms and the results of follow-up examinations, a possible cause of effusion was found and a specific diagnosis was made in 26 (49%) patients. The diagnoses are shown in Table 1.
Diagnoses of parapneumonic effusion and heart failure were made based on the criteria specified in the Patients and Methods section. In one patient, a diagnosis of tuberculosis-related effusion was given when uterine and peritoneal tuberculosis were found and pleural fluid regressed following anti-tuberculosis therapy. Patients diagnosed with pulmonary embolism showed no pathology on computed tomography angiography (which was required prior to VATS), but an embolism was identified in subsequent examinations. One patient had intense eosinophilic infiltration in the pleural biopsy sample, leading to a diagnosis of Churg–Strauss syndrome in view of paranasal sinus anomaly, eosinophilia in peripheral blood, and a history of asthma. In this patient, there was no recurrence of effusion after systemic corticosteroid therapy. Fluid re-accumulation after VATS was seen in another patient, but this was resolved after valproic acid which the patient had been using was discontinued. The diagnosis of this patient was drug-induced effusion.
In 2 patients, diagnosis was malignant mesothelioma. After their initial VATS procedures, re-accumulation of effusion was observed. One of these patients was diagnosed on the basis of pleural fluid cytology performed 11 months after the VATS intervention; the other patient was diagnosed on the basis of a biopsy obtained by VATS 24 months after the first VATS intervention.
VATS is known to be non-diagnostic in patients with malignant mesothelioma. The false-negative rate of VATS for patients with EPE in this study was 3.7%. We examined factors that may have affected the diagnosis of malignancy in this patient group. A weak (r=0.29) but significant correlation was found between age and the possibility of malignancy (P=0.04). Both patients with malignant mesothelioma were men; however, sex was not associated with malignancy (r=0.1, P=0.47). The two patients with malignant mesothelioma did not initially have chest pain, so pain was not associated with a diagnosis of malignant mesothelioma (r=−0.23, P=0.21). A moderate (r=0.43) and significant (P=0.004) correlation was found between the volume of pleural fluid at the initial examination and a diagnosis of malignancy. Smoking was not a risk factor for diagnosis of malignant mesothelioma (r=0.19, P=0.19). The relationships between the initial appearance of fluid and malignant mesothelioma, as well as the other diagnoses, are shown in Table 2. The fluid initially appeared serous in both patients who were diagnosed with malignant mesothelioma. The distribution of the appearance of the initial fluid samples of patients with malignant mesothelioma and with other diagnoses was not significantly heterogeneous (P=0.56).
The LDH level in the initial pleural fluid samples was not an indicator of malignant disease risk (r=−0.24, P=0.14).
Effusion did not recur after the VATS procedure in 45 patients during follow-up. Effusion re-accumulation was encountered in 8 patients. The diagnoses of these patients are shown in Table 3.
A correlation analysis between the development of fluid re-accumulation and the diagnosis of malignant mesothelioma showed a moderate correlation (r=0.47), which was highly significant (P=0.0001).
DiscussionIn over 90% of patients with pleural effusion, diagnostic examinations, including VATS biopsy, allow the cause of the effusion to be identified.4–7 However, in a minority of patients, no specific pleural changes are found. In this case, the conclusion that effusion does not have a specific cause may not always be correct. Some studies have reported that 25–91% of undiagnosed patients have no underlying disease.7 However, in our study, a specific disease causing effusion was identified during follow-up in approximately half of the undiagnosed patients with pleural effusion (26/53 patients, 49%). The majority of diseases diagnosed during follow-up were benign. These results are in line with previous reports,7–9 in which benign diseases, mainly heart failure and parapneumonic effusion, were the most frequently identified causes of pleural effusion.9 Although pleural effusion associated with heart failure is expected to be transudative, the fluid may become exudative in patients receiving diuretics, since these compounds concentrate proteins and other components of pleural transudates in heart failure patients.10 No histological changes were observed in the pleura of these patients, so specific disease findings in VATS pleural biopsy samples would be unlikely. An invasive diagnostic procedure, such as that performed in our study, is indicated in cases where malignancy cannot be excluded clinically and radiologically.11 As in some other cases of undiagnosed effusion, the underlying cause does not always lead to specific histopathological changes in the pleura itself. For example, only nonspecific pleuritis can be detected in the biopsy samples of patients with parapneumonic effusion. Specific changes in the pleura are not expected in cases of pulmonary embolism, Churg–Strauss syndrome (although eosinophilic inflammation in the subpleural and interlobular connective tissue and lymphatic luminal dilation have been reported in Churg–Strauss syndrome biopsy specimens12,13) or drug-related effusion. In the case of tuberculosis peritonitis, which in this study was found to be a cause of effusion, the reaction occurring in the pleura may cause effusion in the absence of granulomatous inflammation.14 In such cases, biopsy samples obtained by VATS are not expected to be diagnostic. Thus, the diagnosis of “nonspecific pleuritis” obtained using VATS in these patients should not be considered a false-negative.
When the diagnoses obtained in this study during follow-up were reviewed, diseases other than malignant mesothelioma could not be recognized by examining the pleural biopsy samples. Only malignant mesothelioma was encountered as a cause of histological impairment in the pleura. The false-negative rate of VATS for malignant mesothelioma was 3.7%.
Subsequent diagnosis of malignant disease in patients with undiagnosed effusion has been reported in up to 25.5% of cases.15 In contemporary studies, the frequency of malignant disease during follow-up in patients lacking a specific diagnosis despite VATS is between 2.1%16 and 5%.9
Malignant mesothelioma is the malignancy most frequently diagnosed during follow-up of patients with undiagnosed pleural effusion.8,9,16,17 In pleural malignant mesothelioma, pleural involvement is uniform, but the appearance of the pleura varies. The typical appearance includes extensive macronodules in the pleura. However, in some patients, gross pleural findings may appear completely normal. Extensive fibrosis may be predominant in other patients. These factors can complicate the diagnosis of malignant mesothelioma by VATS18,19 and may explain why VATS was not diagnostic for patients with malignant mesothelioma in our series.
When we reviewed the factors that increased the likelihood of underlying malignant disease in patients with pleural effusion who could not be diagnosed despite undergoing invasive interventions including VATS, we found that the appearance of the pleural fluid was not significant. Malignant diseases of the pleura generally result in hemorrhagic effusion.20 In our series, the underlying disease in patients with hemorrhagic pleural effusion was heart failure in 2 patients and parapneumonic effusion in 5. In contrast, fluid with a serous appearance does not rule out malignant disease. Nor does biochemical analysis of the fluid indicate malignant disease, as demonstrated here and in an earlier study.17 We found that recurrence of pleural effusion during follow-up might be associated with malignant disease. Although pleural effusion may re-accumulate for reasons other than the presence of malignant disease, recurrence during follow-up warrants additional examination or, if necessary, further VATS. Another risk factor for malignant disease is the presence of an initially large volume of pleural fluid. Such patients may require closer monitoring.
In this study, we found no cause for effusion in about half of patients, and no findings were made that suggested the presence of a specific disease in these patients during follow-up. Although these patients were classified as having idiopathic pleuritis, their actual condition was unknown, and this is a possible limitation of the study. Idiopathic pleuritis describes a situation in which the underlying disease is unknown, and this can be due to many factors. Some patients may present several difficult-to-identify causes of effusion, such as connective tissue diseases and viral or mycoplasma infections,21 as we observed in some of our cases.
In conclusion, no specific disease could be diagnosed in a number of patients despite invasive interventions including VATS. The diagnosis in most patients with EPE is benign disease. However, malignant disease is also a possibility, and these patients should be closely monitored. The characteristics of pleural fluid should not be used as a criterion to discontinue follow-up. The likelihood of malignant disease increases in the presence of effusion that occupies more than half of the hemithorax at the time of initial examination, and that re-accumulates during follow-up. Repetition pleural sampling should be considered in such patients.
Authors’ ContributionsGG made substantial contributions to conception and design, data collection and drafting the manuscript.
AO performed data collection and analysis.
MZG performed data collection, contributed to study conception and design, and statistical analysis.
AS contributed to study design and statistical analysis.
ID performed data collection and analysis.
GC performed data collection and analysis.
VY performed data collection and analysis.
SA made substantial contributions to conception and design.
Conflict of InterestAll the authors declare that they have no financial relationship with any biotechnological, manufacturer or pharmaceutical company, or any other commercial entity that has an interest in the subject matter or materials discussed in the manuscript.
Please cite this article as: Gunluoglu G, Olcmen A, Gunluoglu MZ, Dincer I, Sayar A, Camsari G, et al. Resultados del seguimiento a largo plazo de pacientes con derrame pleural no diagnosticado. Arch Bronconeumol. 2015;51:632–636.