The prognosis of lung cancer (LC) correlates directly with the stage of the disease at the time of diagnosis.
Material and methodsWe performed low-dose CT (LDCT) in asymptomatic individuals ≥50years old, smokers or former smokers of ≥10 pack-years, with no history of cancer. We followed an evaluation algorithm, according to the size and morphology of the nodules. The appropriate treatment for the LC diagnosis was given and patients were followed up for 5years.
ResultsWe studied 4951 individuals (65.4% males) with an average age of 56.89±5.26years; 550 presented nodules. Of the 3891 nodules detected, 692 (19.57%) were considered positive, and 38 tumors (36 LC) were identified. In the annual follow-up, nodules were found in 224 subjects, 288 (7.91%) of which were positive (13 LC). In 80%, the study was performed with LDCT, and biopsy was indicated in 5.8% (baseline) and in 7.6% (annual) of the positive nodules. Prevalence was 0.89 and incidence was 0.1%. The sensitivity, specificity, PPV and NPV in the baseline study were 92.31, 89.54, 6.55 and 99.93%, respectively, and in the annual study, they were 76.92, 95.7, 4.52 and 99.94%, respectively. A total of 52 tumors were detected (49 LC), 25 (52.08%) in stage I. The 5-year overall survival rate for LC was 58.5% and cancer-specific survival was 67.1% (75.8% in surgical patients).
ConclusionLDCT integrated into an elaborate nodule detection and evaluation program is a useful tool for diagnosing early-stage LC.
El pronóstico del cáncer de pulmón (CP) está relacionado directamente con el estadio de la enfermedad al diagnóstico.
Material y métodosRealizamos TC de baja dosis (TCBD) a personas asintomáticas ≥50años, fumadores o exfumadores de ≥10 paquetes-año, sin antecedentes oncológicos. Seguimos un algoritmo de evaluación según el tamaño y la morfología de los nódulos. En los CP diagnosticados se estableció el tratamiento adecuado y el seguimiento fue de 5años.
ResultadosEstudiamos 4.951 personas (65,4% varones) con una media de edad de 56,89±5,26años; 550 presentaron nódulos. De 3.891 nódulos detectados, 692 (19,57%) fueron considerados positivos, hallando 38 tumores (36 CP). En el estudio anual, 224 sujetos mostraban algún nódulo, siendo 288 (7,91%) positivos (13 CP). En el 80% el control se realizó con TCBD y se indicó biopsia en el 5,8% (basal) y 7,6% (anual) de los nódulos positivos. La prevalencia fue del 0,89 y la incidencia del 0,1%. La sensibilidad, la especificidad, el VPP y el VPN en el estudio basal fueron del 92,31, del 89,54, del 6,55 y del 99,93%, respectivamente, y en el anual, del 76,92, del 95,7, del 4,52 y del 99,94%, respectivamente. Se detectaron 52 tumores (49 CP), 25 (52,08%) en estadio I. La supervivencia global de los CP fue del 58,5% a los 5 años, y la supervivencia cáncer específica, del 67,1% (75,8% en los pacientes quirúrgicos).
ConclusionesLa TCBD integrada en un programa elaborado de detección y evaluación de nódulos es una herramienta útil para diagnosticar CP en estadio precoz.
Lung cancer (LC) is one of the most common types of cancer and carries the highest mortality burden, and as such is a significant health problem. Overall survival at 5 years is under 16%, and prognosis is directly related to disease stage at time of diagnosis. Primary prevention of smoking is key to reducing the incidence of LC, and the largest number of cases are diagnosed among former smokers. Even with this primary prevention and recent therapeutic advances, early disease diagnosis is essential if mortality is to be reduced.
The International Early Lung Cancer Action Program (I-ELCAP), a non-randomized study, already reported in 2006 that 85% of cancers were diagnosed in stage I, with an estimated 10-year survival of 88%1 (rising to 92% if the LC was resected within 1 month of diagnosis). The National Lung Screening Trial (NLST) is the first randomized screening study in high-risk individuals to show a reduction in LC mortality of 20.3% in the group that underwent screening with low-dose computed tomography (LDCT) compared to chest X-ray, and a 6.7% reduction in overall mortality.2 These results led the US Preventive Services Task Force (USPSTF) in 2014 to recommend screening in high-risk individuals, with a 2B degree of evidence.3 In 2015, the European Society of Radiology (ESR) and the European Respiratory Society (ERS) voiced their support for screening programs.4 The International Association for the Study of Lung Cancer (IASLC) has recently recommended the implementation of screening based on the results of the NELSON study (NEderlands-leuvens Longkanker Screenings ONderzoek) presented at the 19th World Conference of IASLC Lung Cancer in Toronto. This report confirms that screening with LDCT reduces LC mortality by 26% in men and 61% in women.
In Spain, the Clínica Universitaria de Navarra,5 a member of I-ELCAP, has accumulated extensive experience and made important contributions to screening strategies, including evidence for the use of positron emission tomography (PET),6 the value of emphysema and chronic obstructive pulmonary disease (COPD),7 and the creation of risk prediction models.7,8
In line with opinions published by the Spanish Society of Pulmonology and Thoracic Surgery and other societies on the implementation of screening in 2017,9 we intend to demonstrate that LDCT integrated in a structured protocol is useful for the detection of early-stage, potentially curable LC.
Materials and MethodsStudy PopulationIndividuals entering the Fundación IVO LC screening program between June 2008 and December 2012 were included, forming part of an international cohort in the program I-ELCAP. They met the following criteria: age≥50 years, smokers or former smokers with an accumulated habit of at least 10 pack-years, with no personal history of oncological processes (except basal cell carcinomas of the skin) and no symptoms suggestive of LC. All participants were volunteers (some had been referred by their primary care physician, while most had heard of the program from other participants). After receiving information about the study and the risk of the diagnostic techniques, they signed a consent form approved by the hospital ethics committee. The follow-up of diagnosed cases of LC ended on 31 December 2016.
Diagnostic AlgorithmAll CT scans were performed with 16-slice multidetector spiral CT (Siemens Emotion 16, Erlangen, Germany). Images were acquired with low radiation dose parameters (≤120kVps and ≤30mAs) with CT dose index volume <2.13mGy and dose length product (DLP) of between 80 and 100mGycm. Slice thickness for pulmonary parenchyma was 1mm, and 5mm for mediastinum.
All participants underwent a baseline CT and at least 1 more CT at 1 year. Following the I-ELCAP diagnostic protocol (www.ielcap.org), any lung nodules and other findings in the chest or upper abdomen were identified and characterized. The baseline study was considered to be positive if solid or partially solid noncalcified nodules (NCN)≥5mm or non-solid nodules≥8mm were observed. In that case, patients were referred for LDCT monitoring at 3 months, PET-CT, or biopsy, depending on lesion size or lesions highly suggestive of malignancy. If infection was suspected, antibiotic treatment was recommended and LDCT repeated at 1 month. If partial or complete resolution of the infection was observed, the next scan was performed at 1 year. Cases whose baseline scan was negative were scheduled for the next LDCT at 1 year.
The emergence of new nodules, growth of existing lesions, and all parameters of the initial LDCT were evaluated in the 1-year follow up. The appearance of a solid or partially solid nodule≥3mm, a non-solid nodule≥8mm, or a solid endobronchial nodule≥5mm was considered positive. Solid nodules<3mm or non-solid nodules<8mm were considered semi-positive, and 1-year LDCT was scheduled. When solid nodules measuring 3–5mm were observed, LDCT monitoring was performed at 6 months. Antibiotic treatment was recommended if images suggestive of infection were visualized. If no changes or new nodules were observed, 1-year LDCT was indicated. Nodule growth was defined as an increase in size, solid component, or doubling time. If biopsy was required, the best technique according to the location of the lesion was selected. The most commonly used was CT-guided transthoracic biopsy.
In cases in which a diagnosis of LC was reached, an extension study was performed to stage the disease, and to determine if the tumor was resectable and the patient could undergo surgery. Elective treatment was decided on an individual basis, in consensus with the multidisciplinary committee of our hospital. In this study, we used the 8th edition of the TNM classification.10
Statistical AnalysisThe various demographic and radiological variables and LC characteristics, etc. were analyzed using SPSS for Windows® version 22. A descriptive and comparative analysis was performed, using the Chi-squared (χ2) method or Fisher's exact test, Student's t test, Mann–Whitney U test, and ANOVA, or the Pearson's correlation test for continuous variables. The Kaplan–Meier method11 and the log-rank test12 were used to calculate survival.
The prevalence rate included all cases of LC identified in the baseline screening, and any nodules diagnosed during the second scan that were found to be dominant. The incidence rate was calculated from newly identified lesions. True positive (TP) cases were dominant LC nodules at any stage or dominant nodules progressing to stage I in the subsequent follow-up. False negatives (FN) were dominant nodules that were determined in the 1-year screening to be LC>stage I and any interim diagnosis of LC. TP and FN values were the same for both LC cases and cases with nodules, because while an individual can have more than 1 nodule, and as none of our cases had LC in multiple sites, cases were diagnosed as a single LC. False positives (FP) were nodules or patients with radiologically positive nodules in whom a diagnostic procedure was indicated, but which were not LC. True negatives (TN) were nodules that did not fulfill radiological criteria or individuals who did not have nodules or who, if nodules were identified, these remained stable or disappeared in the 1-year follow-up. Sensitivity (S), specificity (SP), diagnostic precision (DP), positive and negative predictive values (PPV and NPV), likelihood ratio, and positive and negative probability ratios (PPR and NPR) were calculated to characterize the validity of the test.
ResultsWe included 4951 subjects in the study (Table 1). Of these, 65.4% were men; mean age was 56.89±5.26 years, and median age 56 years, with a range of 50–79. Mean tobacco consumption was 37.45±23.41 pack-years for men and 29.06±16.73 for women. In the baseline assessment, 2238 (45.8%) were former smokers.
Characteristics of Study Population.
| Sex | |
| Men | 3237 (65.4%) |
| Women | 1714 (34.6%) |
| Age, years | |
| 50–60 | 3905 (78.87%) |
| 61–70 | 942 (19.02%) |
| >70 | 104 (2.01%) |
| Smoking | |
| Active smokers | 2713 (54.8%) |
| Former smokers | 2238 (45.8%) |
| Pack-years | |
| Total | 34.53 |
| Men | 37.45 |
| Women | 29.06 |
| Family history of LC | |
| Yes | 933 (18.84%) |
| No | 3913 (79.04%) |
| Not known | 105 (2.12%) |
| Contact with asbestos | |
| Yes | 417 (8.42%) |
| History of respiratory disease | |
| Pneumonia | 195 (3.94%) |
| Emphysema | 253 (5.11%) |
| Asthma | 159 (3.21%) |
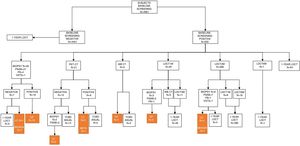
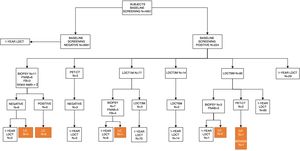
In the baseline assessment, CT was negative in 4401 (88.89%) subjects, and 550 (11.11%) showed 1 or more nodules that were identified as positive. Of 3891 nodules detected, 692 met the criteria of positivity according to the I-ELCAP protocol. The follow-up schedule was determined on the basis of these results. Most cases (80.20%) were referred for a repeat LDCT as the first course of action, and in 89.36% the scan was performed at 3 months. Histological studies were performed in 23 cases (3.32%), PET-CT was performed in 2 (3.03%) and bronchoscopy in 5 (Fig. 1). Biopsy was indicated in 2 cases, despite a negative PET, given the small size of the nodules and the possibility of FP in the PET.
In the 1-year scan, excluding the 36 subjects diagnosed with pulmonary tumors, LDCT was negative in 4691 (95.44%) participants, and 224 (4.56%) were positive; 4081 nodules were detected, of which 288 were positive. In 79.86% of the positive cases, LDCT follow-up at 1 month was indicated as a first option in 46.08%, in 47.83% at 6 months, and in 44 cases at 1 year. FNAB was indicated in 6 patients, PET-CT in 3, and bronchoscopy in 2. Surgical resection was indicated in only 1 patient (Fig. 2).Forty biopsies were carried out at baseline (32 FNAB, 6 bronchoscopies, 1 video-assisted surgery, and 1 exploratory thoracotomy), and 21 at the 1-year scan (12 FNAB, 7 bronchoscopies, and 2 thoracotomies). In the 44 FNABs performed, 6 patients developed pneumothorax as the only complication, of which only 2 required drainage. Forty (5.8%) of the 692 nodules classified as positive at baseline required invasive procedures. At the 1-year scan, 7.3% (21/288) of nodules were positive. We diagnosed 38 pulmonary tumors at baseline screening, including 36 LCs, and 14 in the 1-year scan, of which 13 were LC. Of these, 5 patients were diagnosed on the basis of newly identified nodules. The prevalence rate was estimated at 0.89%, and incidence at 0.1%.
To evaluate the screening program and LDCT as a diagnostic tool, we calculated S, SP, PPV, NPV, PPR, and NPR for individuals and for nodules (Tables 2 and 3).
Evaluation of Diagnostic Tests in Baseline LDCT Screening.
| Individuals | Nodules | |||
|---|---|---|---|---|
| TP | 36 | 36 | ||
| TN | 4398 | 2841 | ||
| FP | 514 | 656 | ||
| FN | 3 | 3 | ||
| S (95% CI) | 92.31 | 78.03–97.99 | 92.31 | 78.03–97.99 |
| SP (95% CI) | 89.54 | 88.64–90.37 | 81.24 | 79.90–82.51 |
| PPV (95% CI) | 6.55 | 4.69–9.03 | 5.20 | 3.72–7.20 |
| NPV (95% CI) | 99.93 | 99.78–99.98 | 99.89 | 99.66–99.97 |
| DP (95% CI) | 89.56 | 88.66–90.39 | 81.36 | 80.03–82.63 |
| PPR (95% CI) | 8.82 | 7.81–9.97 | 4.92 | 4.39–5.53 |
| NPR (95% CI) | 0.09 | 0.03–0.25 | 0.09 | 0.03–0.28 |
Evaluation of Diagnostic Tests in 1-Year LDCT Scan.
| Individuals | Nodules | |||
|---|---|---|---|---|
| TP | 10 | 10 | ||
| TN | 4691 | 3349 | ||
| FP | 211 | 278 | ||
| FN | 3 | 3 | ||
| S (95% CI) | 76.92 | 45.89–93.84 | 76.92 | 45.89–93.84 |
| SP (95% CI) | 95.70 | 95.08–96.24 | 92.34 | 91.41–93.17 |
| PPV (95% CI) | 4.52 | 2.32–8.41 | 3.47 | 1.77–6.49 |
| NPV (95% CI) | 99.94 | 99.80–99.98 | 99.91 | 99.72–99.98 |
| DP (95% CI) | 95.65 | 95.03–96.19 | 92.28 | 91.35–93.12 |
| PPR (95% CI) | 17.87 | 12.90–24.75 | 10.04 | 7.30–13.80 |
| NPR (95% CI) | 0.24 | 0.09–0.65 | 0.25 | 0.09–0.67 |
In the baseline screening, 692 (19.57%) were radiologically positive, and of these, 656 (94.8%) were considered FP, after the exclusion of LC. Taking into account all noncalcified nodules (NCN) (3536), the rate of FP was 18.55%. In the 1-year scan, 96.52% of the nodules classified as positive, and 7.64% of all NCNs, were FPs.
We identified a total of 52 tumors: 48 LC (Table 4), 1 metastatic melanoma (unknown primary), and 3 benign tumors (tuberculoma, histiocytosis X, aspergilloma). A total of 52.09% of the tumors were diagnosed in stage I (Table 5). Forty (76.92%) LCs were treated surgically. The median follow-up was 3.67 years (0.01 to 7.99).
Histological Types of LC Detected.
| Carcinoid tumor | 2 | 4.17% |
| Squamous cell carcinoma | 3 | 6.25% |
| Adenocarcinoma | 38 | 79.17% |
| Poorly differentiated | 3 | |
| Mixed | 9 | |
| Predominantly papillary-acinar | 2 | |
| Predominantly solid | 6 | |
| Predominantly acinar | 8 | |
| Predominantly solid-acinar | 4 | |
| With enteric differentiation | 1 | |
| With clear cell differentiation | 1 | |
| Adenocarcinoma in situ | 4 | |
| Adenosquamous carcinoma | 2 | 4.17% |
| Undifferentiated carcinoma | 1 | 2.08% |
| Small cell carcinoma | 2 | 4.17% |
| Total | 48 | 100% |
Staging of LC Detected.
| IA | T1aN0M0 (18) | 20 | 41.67% |
| T1BN0M0 (2) | |||
| IB | T2aN0M0 (5) | 5 | 10.42% |
| IIA | T1aN1M0 (1) | 2 | 4.16% |
| T2aN1M0 (1) | |||
| IIB | T3N0M0 (3) | 3 | 6.25% |
| IIIA | T1aN2M0 (2) | 10 | 20.83% |
| T1BN2M0 (3) | |||
| T2aN2M0 (1) | |||
| T2BN2M0 (3) | |||
| T4N1M0 (1) | |||
| IIIB | TxN3M0 (1) | 5 | 10.42% |
| T1aN3M0 (1) | |||
| T1bN3M0 (1) | |||
| T2aN3M0 (2) | |||
| IV | M1a (1) | 3 | 6.25% |
| M1b (2) | |||
| Total | 48 | 100% |
Overall survival of the detected LCs was 58.5% at 5 years, with 67.1% cancer-specific survival (75.8% in the surgical patients and 41% in the non-surgical patients). Five-year survival in stage IA LCs was 89.4% and 80% in stage IB tumors.
DiscussionThis study shows that LDCT is a valid tool for LC screening as part of a comprehensive program for early detection of lung nodules.
Existing studies vary widely in terms of sample selection criteria and the design of protocols for evaluating the nodules. Like us, many research groups included voluntary participants. The NLST13 authors speak of the “healthy volunteer effect”, with individuals who are more committed to both their health and the program.14 It is clear that selecting higher-risk patients and cohorts with greater disease prevalence would increase the yield of screening. For this reason, the NELSON15 investigators selected their population from individuals who responded to a questionnaire; other studies took into account the existence of COPD/emphysema,7 and others used risk prediction models evaluating multiple factors that without doubt improve sensitivity and PPV.16,17
The proportion of nodules considered radiologically positive in our study was 19.57% in the baseline screening and 7.91% in the 1-year scan. Newly identified nodules are less frequent than dominant nodules, but are more likely to progress to LC. Henschke et al.,1 using the same criteria, found a rate of positive nodules of 13%. In the NLST study,18 the rate of positive nodules in the first round of screening was 27.3%. The NELSON study15 estimated a rate of 2.6%, but if we take into account the nodules defined as indeterminate which underwent LDCT follow-up at 3 months, the rate is 19.2%. In our study, 94.8% and 96.52% of the nodules defined as positive in the baseline and 1-year scans, respectively, could be considered FP, although with respect to the total number of NCNs, the FP rate was 18.55% and 7.64%, respectively. The NLST study found an FP rate of 96%.18,19 In the NELSON study, in the first 3 rounds, excluding indeterminate nodules, 200 LC were identified among the 493 positive tests, i.e., 64.3% of positive nodules can be considered FPs. The authors estimated a rate of 3.86%.20 It is clear, then, that the rate of FP depends largely on how a positive nodule is defined. In the opinion of the I-ELCAP and NELSON investigators, a nodule that does not grow should not be regarded as positive.
Most positive results only needed to be followed up with imaging procedures. Invasive procedures were required in 40 (5.8%) of the 692 nodules classified as positive on baseline LDCT, and in 20 (7.3%) of the 288 classified as positive in the 1-year scan. Results were positive in 95% and 66% of biopsies performed, showing that the evaluation protocol can accurately select patients who really require a biopsy. In the 1-year scan, biopsy was indicated when there was growth of existing nodules or appearance of new nodules (the majority of them, 275). Newly identified nodules were less common than dominant nodules, but are more likely to be malignant. This was probably the reason why a greater number of biopsies were negative (or not useful for diagnosis) in the 1-year scan. In any case, iatrogenic complications were rare, and none were severe.
As in other observational series, the prevalence rate is much higher than the incidence rate. In our study, the prevalence rate (0.89%) was close to that reported in the P-ELCAP study (1%),5 in Veronesi et al.21 (1.1%), and in the I-ELCAP study (1.3%).1 We found a low incidence rate (0.1%) compared to other studies (P-ELCAP,5 1.4%), that was closer to that of I-ELCAP (0.3%)1 or the 1-year detection rate of P-ELCAP (0.41%). These differences may be explained by the different inclusion criteria, or because we estimated the incidence rate only from newly occurring nodules.
S and SP of both the evaluation protocol, taking individuals into account, and the LDCT, taking nodules into account, are very high. NPV is a key parameter in any screening program, and in our case it was 99.9%. PVV, defined as the probability of disease when the test is positive, was around 6.55%, in line with other studies.19,22–25 Only Henschke et al.1,26 found a PPV of 11.5% in the initial screening, that increased to 25% in subsequent rounds. The number of FPs determines a low PPV rate. Given our high S and high NPV, a negative result makes it highly unlikely that the individual has cancer.
DP, understood as the percentage of patients who were diagnosed correctly, was 89.56% and 95.65% in the baseline and 1-year scans, respectively. We used likelihood ratios to determine diagnostic power. Our PPR suggests that it is 9–18 (baseline/1-year) times more likely to obtain a positive result in a patient than in a healthy individual. This PPR in the 1-year scan shows that it is a very powerful strategy that strongly supports the diagnosis. We found an NPR of 0.09 in the baseline screening, indicating that the power of screening for ruling out a diagnosis is high. No similar data have been published from other studies.
Prevalence is, in short, the probability of having disease before screening is performed. PPV would be the post-screening probability of having disease, and lies at around 5%-6.5%. We know the prevalence rate of LC in the population (approximately 45/100000 inhabitants). The resulting ratio is 111–144, meaning that we have multiplied our ability to detect patients with disease by 111–144.
Distribution by LC stages is slightly lower than in other studies: in the NLST and in the NELSON studies, 61.6% and 70.8%, respectively, were stages IA-IB.27 In P-ELCAP,5 73% of LCs were stage I. The percentage of early stage disease increases in successive rounds (results not shown).
Surgery was performed in 76.92% of our cases. In the I-ELCAP, 85% of patients underwent resection, with a 10-year survival of 92% when the intervention was performed within 1 month of diagnosis.1 Cancer-specific survival in our study was 67.1%, and 75.8% in the group undergoing surgery. Other European studies found similar data: Bellomi et al.28 found that 72% of their patients with a diagnosis of LC were in stage I. This group had a 5-year survival rate of 89% and an overall survival rate of 63%. Blanchon et al.29 found an 80% survival rate for LC detected in the group undergoing CT screening, and 88% for LC in stage I. These data are promising if we remember the overall survival of LC in the general population.
One of the main limitations of screening is overdiagnosis, which we cannot evaluate in the absence of a control group. Overdiagnosis is often confused with FPs. FP is a diagnostic error, and overdiagnosis is a prognostic error.30
LDCT integrated into a standardized evaluation protocol with a carefully designed and defined algorithm (with specific inclusion criteria determining the duration and intervals of screening) is a valid tool for screening because it helps diagnose LC at earlier stages. It is important to minimize all limitations before it can be implemented as a populational screening tool.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Martínez Pérez E, de Aguiar Quevedo K, Arrarás Martínez M, Cruz Mojarrieta J, Arana Fernández de Moya E, Barrios Benito M, et al. Diagnóstico precoz del cáncer de pulmón: utilidad de la tomografía computarizada de baja dosis de radiación. Arch Bronconeumol. 2019;55:526–531.