Hypersensitivity pneumonitis (HP) is an interstitial lung disease caused by the inhalation of specific organic antigens or low-molecular weight substances in genetically susceptible individuals. Although small airway involvement is prominent in patients with chronic HP, conventional pulmonary function tests (PFTs) are relatively insensitive to identify it. Thus, the authors aimed to evaluate resistance (R5) and reactance (X5) values at 5Hz on inspiration, expiration, and whole breath, as well as small airway resistance (R5•19) values using a forced oscillation technique (FOT) in patients with chronic HP, and their responses after bronchodilator. In addition, R5 and X5 values according to the presence or absence of mosaic attenuation on computed tomography (CT) were compared.
MethodsPFTs with plethysmography, diffusing capacity of the lungs for carbon monoxide (DLCO) and FOT measurements were performed pre-bronchodilator and post-bronchodilator. High-resolution CT was performed at the same visit, and classified according to the presence or absence of mosaic attenuation. R5 and X5 values were then compared according to the presence or absence of mosaic attenuation on CT.
ResultsTwenty-eight patients with chronic HP (57.1% female; mean age, 56±11.5 years; mean forced vital capacity 57±17% predicted) were evaluated. All patients had low X5 values, reflecting lower lung compliance, and only three (8%) demonstrated elevated R5 (whole-breath) values. No patients exhibited bronchodilator response in R5, X5 and R5•19 values. In patients who exhibited greater extension of mosaic attenuation (n=11), R5 and X5 values could not discriminate those with a greater presence of these areas on CT.
ConclusionsThe results suggest that FOT does not help to additionally characterise concomitant small airway involvement in patients with chronic fibrotic HP who demonstrate restrictive ventilatory pattern in conventional PFTs. Nevertheless, FOT appeared to better characterise decreased lung compliance due to fibrosis through X5. Bronchodilator therapy did not appear to induce an acute response in chronic HP patients with restrictive disease. The precise role of FOT in subacute HP and obstructive chronic HP, therefore, must be evaluated.
La neumonitis por hipersensibilidad (HP) es una enfermedad pulmonar intersticial causada por la inhalación de antígenos orgánicos específicos o sustancias de bajo peso molecular en individuos genèc)ticamente susceptibles. Aunque la implicación de las vías aèc)reas pequeñas es típica en pacientes con HP crónica, a las pruebas habituales de función pulmonar (PFP) les falta sensibilidad para detectarla. El objetivo fue evaluar los valores de resistencia (R5) y de reactancia (X5) a 5Hz durante la inspiración, la espiración y el ciclo completo, así como los valores de resistencia de las vías aèc)reas pequeñas y las respuestas tras broncodilatador. Para ello se utilizó la tèc)cnica de oscilación forzada (TOF) en pacientes con HP crónica. Además, se comprobaron los valores R5 y X5, de acuerdo con la presencia o ausencia de patrones de atenuación en mosaico mediante tomografía computarizada (TC).
Mèc)todosSe evaluaron las PFP con pletismografía, la capacidad de difusión pulmonar para el monóxido de carbono (DLCO) y la TOF antes y despuèc)s de broncodilatador (pre- y posbroncodilatador, respectivamente). La TC de alta resolución se realizó en la misma visita, y los resultados se clasificaron en función de la presencia o ausencia de patrones de atenuación en mosaico.
ResultadosSe evaluaron 28 pacientes con HP crónica (57,1% mujeres; edad media: 56±11,5 años; capacidad vital forzada media estimada: 57±17%). Todos los pacientes tuvieron valores X5 bajos, indicativo de una distensibilidad pulmonar baja, y solo 3 (8%) presentaron valores elevados de R5 (respiración completa). Ningún paciente exhibió respuesta broncodilatadora en valores R5, X5 y R5-19. Los valores de R5 y X5 no permitieron discriminar a aquellos pacientes que presentaron patrón de atenuación en mosaico extenso en la TC (n=11).
ConclusionesLos resultados sugieren que la TOF no proporciona información extra en la caracterización de la implicación concomitante de las vías aèc)reas pequeñas en pacientes con HP crónica que presentan patrones ventilatorios restrictivos en PFP convencionales. Sin embargo, la TOF parece permitir una mejor caracterización de la disminución de la distensibilidad pulmonar debido a fibrosis a travèc)s del valor X5. La terapia broncodilatadora no indujo respuesta aguda en pacientes con HP crónica y enfermedad restrictiva. Por tanto, se debe evaluar el papel específico de la TOF en la HP subaguda y la HP obstructiva crónica.
Hypersensitivity pneumonitis (HP) is an interstitial lung disease (ILD) with variable clinical symptoms caused by the inhalation of specific organic antigens or low-molecular weight substances in genetically susceptible individuals.1•4 Chronic HP represents the final stage, in which prolonged antigenic exposure causes fibrosis.
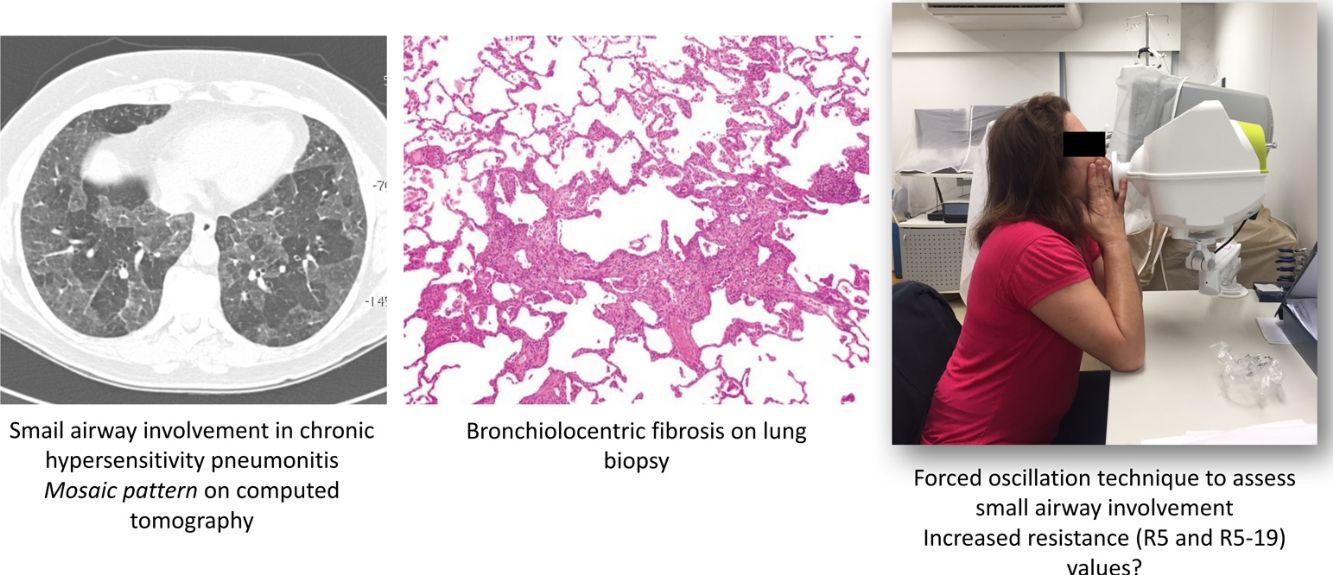
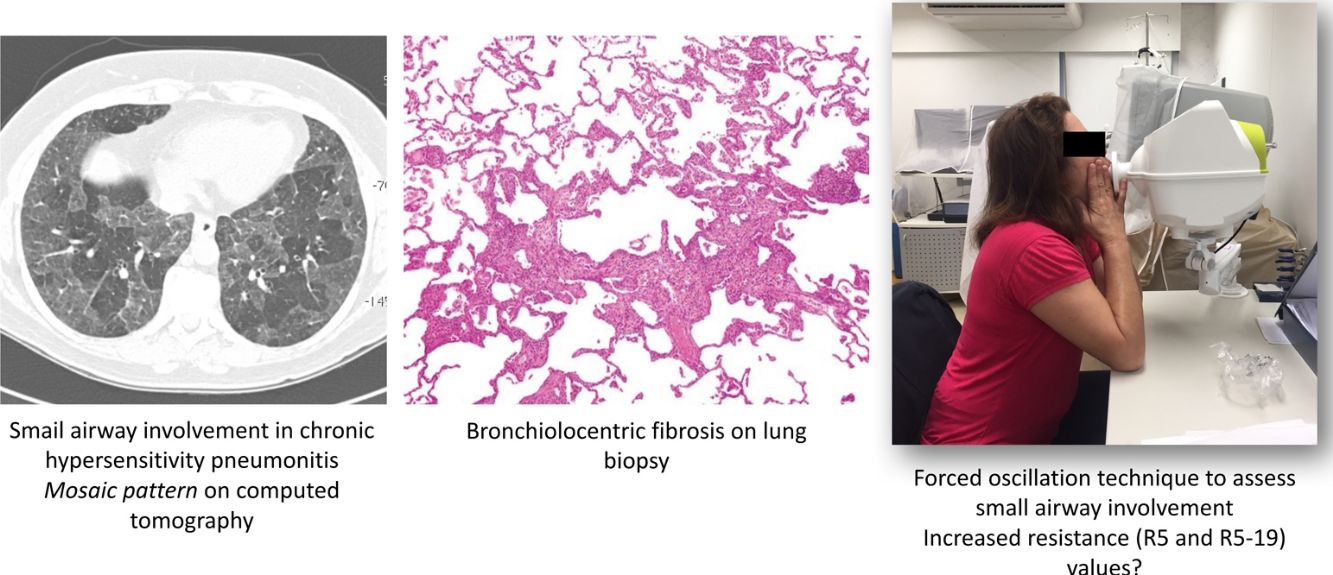
Unique histopathological features of HP include some degree of abnormality within and/or around the small airways of all patients with HP.5 On computed tomography (CT), small airway disease in chronic HP is revealed by findings of mosaic attenuation pattern(s), air trapping on expiratory scans, centrilobular ground-glass opacities, and the absence of predominance in inferior lobes.6•8
However, conventional pulmonary functional tests (PFTs) are relatively insensitive to detect small airway involvement in the presence of concomitant fibrotic ILD,9•11 explaining why obstruction is found only in a small percentage of patients with chronic HP.2,12
The forced oscillation technique (FOT) is a promising tool that enables lung function measurements using sinusoidal sound waves of single frequencies generated by a loud speaker and passed into the lungs during tidal breathing.13,14 FOT has demonstrated greater sensitivity in detecting peripheral airway obstruction.14,15 The output is a measure of respiratory impedance (Zrs), which includes the respiratory resistance (Rrs) and reactance (Xrs) measured over a range of frequencies.14 Resistance comprises the pressure-flow relationship, while reactance comprises the elastic elements and the inertial forces arising from the acceleration of tissues and gas in the respiratory system.13 Higher frequencies (>20Hz) travel shorter distances, such as in the large airways, while lower frequencies (<15Hz) travel deeper into the lung and reach the small airways.14
FOT has been used for the evaluation of asthma16,17 and chronic obstructive pulmonary disease (COPD)18•20; however, its role in the evaluation of small airway involvement in ILD is relatively incipient.21•26 Increased Rrs values at low frequencies have been found in sarcoidosis,24 silicosis27 and asbestosis,28 while reactance at 5Hz (X5) values are usually low in patients with ILD.22 Recently, Guerrero-Zuñiga and colleagues demonstrated an increased resistance at 5Hz (R5) in 40% of their chronic HP cohort through impulse oscillometry (IOS), suggesting the presence of small airway involvement.29 However, neither FOT measurements using specific frequencies, nor their values after bronchodilator administration, were evaluated. Moreover, X5 and R5 values have not been previously compared according to the presence or absence of air trapping on CT.
We hypothesised that FOT has a higher sensitivity for evaluation of small airway involvement in patients with chronic HP, even in those without obstruction in PFTs. Therefore, the present study aimed to: evaluate resistance (R5) and reactance (X5) values at 5Hz, and small airway resistance (R5•19) using FOT, which could potentially characterise airflow limitation in chronic HP patients; evaluate bronchodilator response after administration of salbutamol; and, finally, compare R5 and X5 values according to the presence or absence of mosaic attenuation pattern suggesting air trapping on CT.
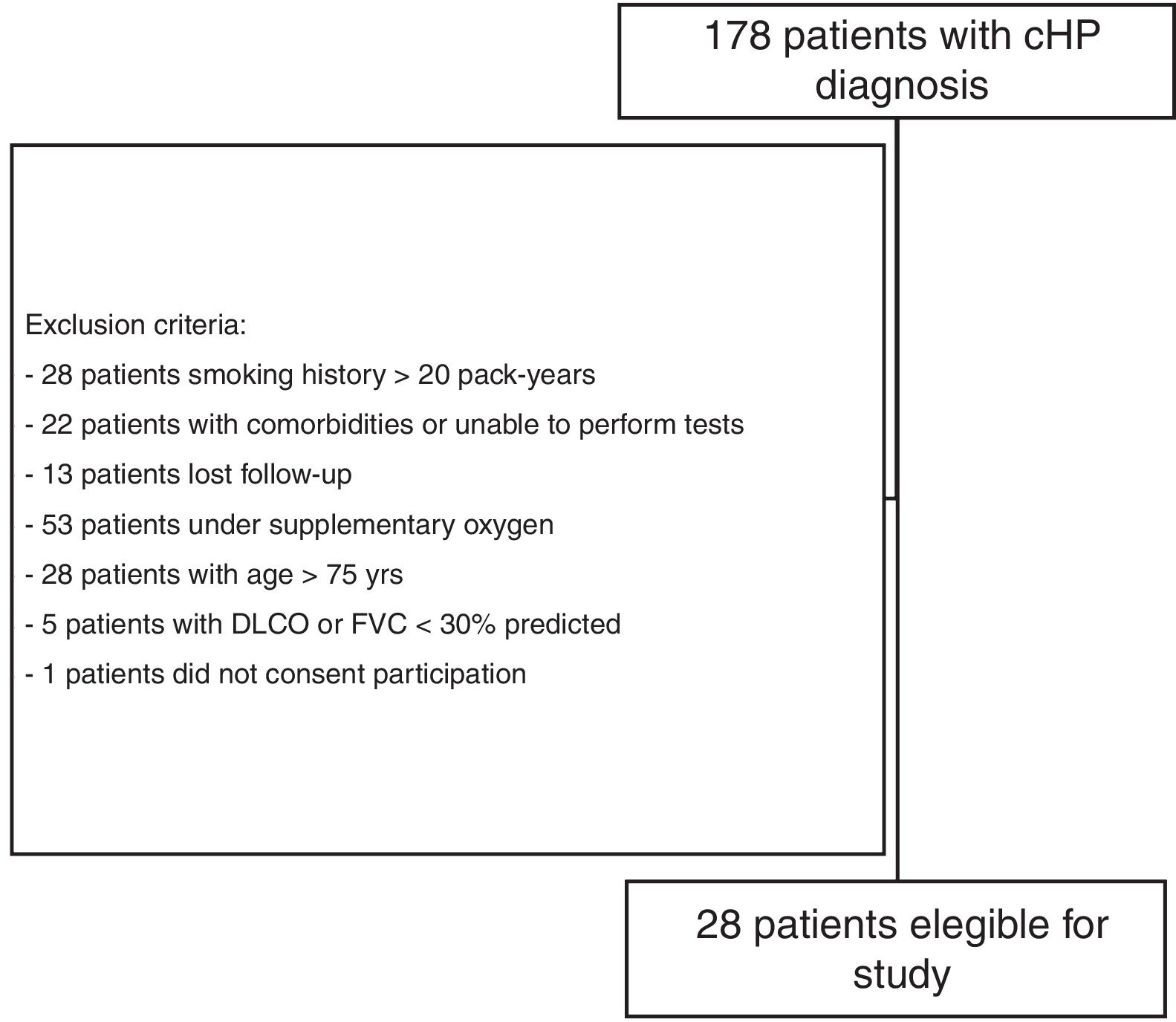
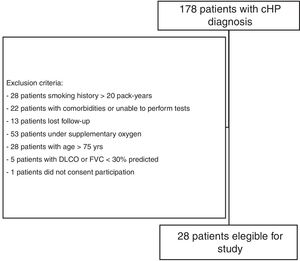
Materials and methodsStudy subjectsThe present investigation was part of a larger comprehensive study evaluating small airway involvement in patients with chronic HP (ClinicalTrials.gov Identifier NCT 02523833) using volumetric CT scans, PFTs, cardiopulmonary exercise test (CPET), and FOT examinations. From September 2015 to March 2017, 178 patients with confirmed diagnosis of chronic HP, who were followed at the ILD outpatient clinic of the Pulmonary Division of the Hospital das Clínicas of the University of São Paulo (São Paulo, Brazil), were prospectively evaluated, and 28 were enrolled (Fig. 1). A diagnosis of chronic HP was defined based on CT findings (fibrotic involvement of the pulmonary parenchyma), known antigenic exposure, exclusion of other possible diagnoses, and compatible histology on transbronchial or open lung biopsy, or bronchoalveolar lavage with lymphocytosis >30%.30 Patients were clinically stable (without hospitalisation, or evidence of disease progression requiring modification of drug regimen) for at least 6 weeks, and were excluded if >75 years of age, had a forced vital capacity (FVC) and/or forced expiratory volume in 1s (FEV1) <30% of predicted, smoked >20 pack-years, had a previous diagnosis of asthma or COPD, or were unable to perform CPET. The study protocol was approved by the local research ethics committee (SDC 3966/13/091), and all patients provided informed written consent.
The sample size was calculated based on the study by Mori and colleagues22 in patients with combined pulmonary fibrosis and emphysema, given that at the time of study design, no previous study had been published in chronic HP and was the scenario that best fit concomitant small airway involvement and fibrosis. Assuming a standard deviation of R5 and X5 values of the study population with a two-tailed alpha error of 5% and 80% power, the number of chronic HP patients required for statistical significance was 28.
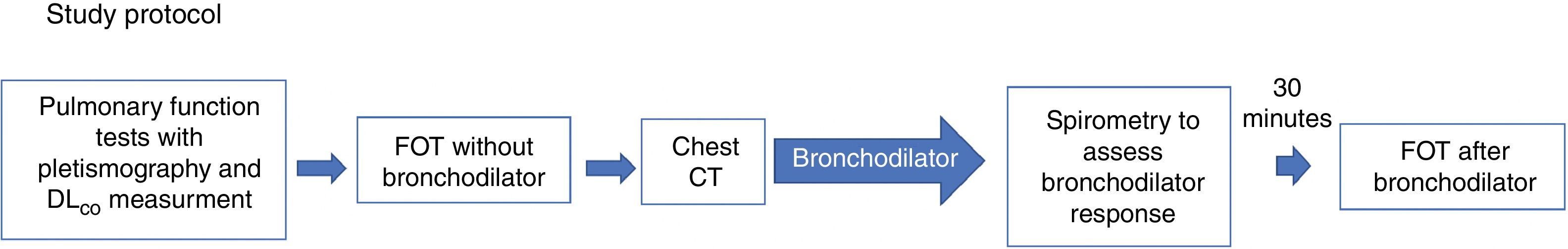
Study protocolThe study protocol is shown in Fig. 2. Patients included in the protocol underwent PFTs with plethysmography and diffusing capacity of the lungs for carbon monoxide (DLCO) measurements, and later, FOT without bronchodilator. PFTs were repeated after bronchodilator use, and FOT after bronchodilator was repeated at least 30min after spirometry. Computed tomography was performed at the same visit.
PFTsPFTs adhered to the European Respiratory Society/American Thoracic Society guidelines.31•33 Spirometry was performed using a calibrated pneumotachograph (Medical Graphics Corp., St. Paul, MN, USA). Lung volumes and DLCO were measured using a body plethysmograph (Elite Dx, Elite Series; Medical Graphics Corp). FVC, FEV1, total lung capacity, residual volume (RV), and DLCO were obtained. Subsequently, all patients were administered salbutamol (400 mcg) and underwent repeated measurement of the variables assessed at baseline spirometry. Predicted values were derived from the Brazilian population.34,35
FOTFOT measurements were obtained according to specific guidelines.36 The device used was the Resmon Pro Full (MGC Diagnostics, Milan, Italy), which was calibrated daily, in accordance with manufacturer's instructions. This equipment simultaneously measures at frequencies of 5Hz, 11Hz and 19Hz. Patients were placed in a sitting position with their head in neutral or slightly extended position, with the mouthpiece of the pneumotachograph positioned between the lips. During measurement, the patient held the malar region and a nasal clip was positioned to prevent air leakage. Patients were instructed to breathe quietly at the level of their residual functional capacity. Manoeuvres with artefacts (swallowing, glottal closure, vocalisation, irregular breathing, or acute hyperventilation) were automatically discarded by the pneumotachograph. Three technically acceptable measurements were performed with approximately 15 respiratory cycles each (1•2min for acquisition). Manoeuvres were considered to be acceptable when intra-individual variability between measurements was between 5% and 15%. The first measurement was discarded as a manoeuvre for the patient to become accustomed to the method and equipment; the mean value obtained from two other valid consecutive manoeuvres was compiled as the final result. For small airway evaluation, reactance (X5) and resistance (R5) values obtained at 5Hz were evaluated at inspiration, expiration, and whole-breath. Additionally, small airway resistance (i.e., total resistance minus the resistance of the central airways [R5•19]) was also evaluated at inspiration. Manoeuvres were repeated after administration of 400mcg salbutamol as a bronchodilator test. Reduction in R5 values >40% were considered to be a positive bronchodilator response.14
CT protocol and classification of low-attenuation areasDetailed information regarding CT acquisition parameters for patients with chronic HP is reported in the Supplemental material.
Quantification of low-attenuation areas on CTMosaic attenuation, when present, was independently classified by a pneumologist (O.M.D.) and a thoracic radiologist (R.C.C., with 16 years tm) experience) who were blinded to the clinical information and histological diagnosis. Based on a previously published classification of low-attenuation areas in chronic HP,7 each patient was classified by counting the number of secondary lobules with decreased attenuation on inspiratory images, as follows:
- •
Class 0: no low-attenuation areas
- •
Class 1 (up to 4 lobules)
- •
Class 2 (≥5 lobules and involving 2•4 lobes)
- •
Class 3 (≥5 lobules in >4 lobes [the lingula being considered a separate lobe]).
Quantification of lobular areas with decreased attenuation and vascularity was limited to the presence in nondependent lung, and away from the superior segment of the lower lobe or the tip of the lingula or right middle lobe, or within areas of severe fibrosis to avoid confounding with lobular areas usually found in normal subjects.37 These four categories were grouped as ‘no mosaic attenuation tm) (i.e., classes 0 and 1) and ‘mosaic attenuation tm) (i.e., classes 2 and 3). Disagreements between classifications were resolved by consensus.
Dyspnoea and quality of life were assessed using the modified Baseline. Dyspnoea Index and the Short Form 36 Health Survey (SF-36) questionnaire, which has been validated for the Brazilian population (18•21).
The level of daily physical activity based on aerobic exercises was evaluated by a specific questionnaire that had been validated previously (22).
Dyspnoea and quality of life were assessed using the modified Baseline. Dyspnoea Index and the Short Form 36 Health Survey (SF-36) questionnaire, which has been validated for the Brazilian population (18•21). The level of daily physical activity based on aerobic exercises was evaluated by a specific questionnaire that had been validated previously (22).
Statistical analysesValues are reported as mean±standard deviation (SD) for normally distributed variables, or as median (25th•75th percentiles) for non-normally distributed variables. The paired t test or the Wilcoxon test were used to compare continuous variables among chronic HP patients for bronchodilator response; unpaired t-tests or Mann•Whitney test were used to compare FOT data among patients according to the presence or absence of low-attenuation areas on CT.
A two-way repeated-measures analysis of variance was used to analyse time course differences of the variables with normal distribution and to e valuate differences between groups. A two-way repeated-measures analysis of variance was used to analyse time course differences of the variables with normal distribution and to e valuate differences between groups.
Statistical significance was set at P<0.05. Statistical analyses were performed using SPSS version 21.0 (IBM Corporation, Armonk, NY, USA) for Macintosh (Apple Inc, Cupertino, CA, USA).
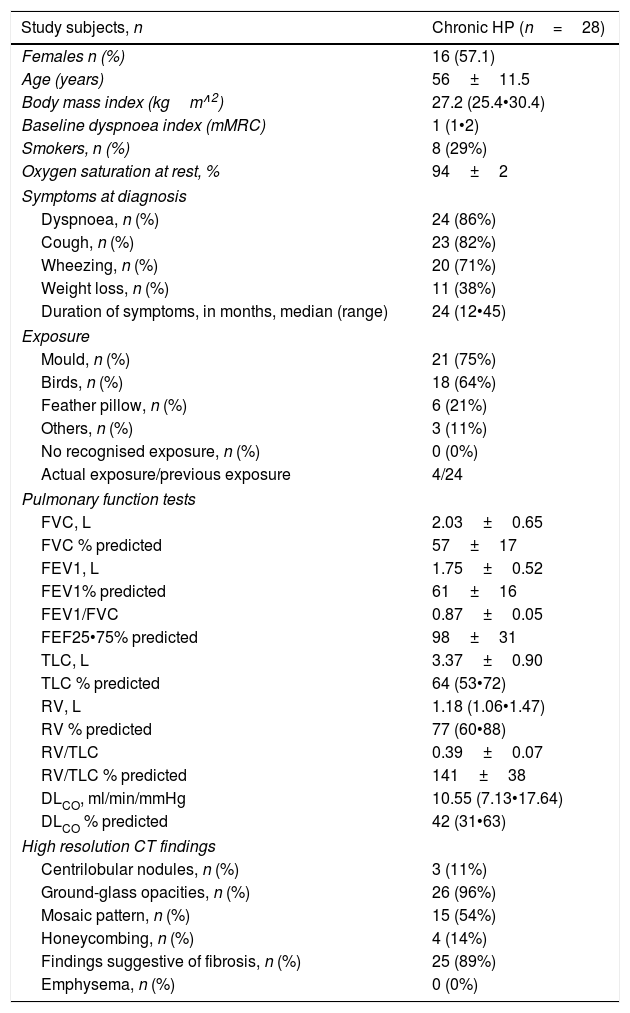
ResultsBaseline characteristics of the patientsThe general characteristics of patients with chronic HP are presented in Table 1. Of the 28 patients included, 16 were female, and the mean age of the entire cohort was 56±11.5 years. The main clinical symptoms were dyspnoea (86%) and cough (82%). Mean FVC (% predicted) was 57±17%, and mean FEV1 was 61±16%. No patients exhibited obstruction on PFTs nor response to bronchodilator. The main CT findings were ground-glass opacities (96%) and those suggestive of fibrosis (reticulation/traction bronchiectasis [present in 89%]). Any extent of mosaic pattern was present in 54% of the patients.
Baseline characteristics of patients with chronic hypersensitivity pneumonitis.
| Study subjects, n | Chronic HP (n=28) |
|---|---|
| Females n (%) | 16 (57.1) |
| Age (years) | 56±11.5 |
| Body mass index (kgm∧2) | 27.2 (25.4•30.4) |
| Baseline dyspnoea index (mMRC) | 1 (1•2) |
| Smokers, n (%) | 8 (29%) |
| Oxygen saturation at rest, % | 94±2 |
| Symptoms at diagnosis | |
| Dyspnoea, n (%) | 24 (86%) |
| Cough, n (%) | 23 (82%) |
| Wheezing, n (%) | 20 (71%) |
| Weight loss, n (%) | 11 (38%) |
| Duration of symptoms, in months, median (range) | 24 (12•45) |
| Exposure | |
| Mould, n (%) | 21 (75%) |
| Birds, n (%) | 18 (64%) |
| Feather pillow, n (%) | 6 (21%) |
| Others, n (%) | 3 (11%) |
| No recognised exposure, n (%) | 0 (0%) |
| Actual exposure/previous exposure | 4/24 |
| Pulmonary function tests | |
| FVC, L | 2.03±0.65 |
| FVC % predicted | 57±17 |
| FEV1, L | 1.75±0.52 |
| FEV1% predicted | 61±16 |
| FEV1/FVC | 0.87±0.05 |
| FEF25•75% predicted | 98±31 |
| TLC, L | 3.37±0.90 |
| TLC % predicted | 64 (53•72) |
| RV, L | 1.18 (1.06•1.47) |
| RV % predicted | 77 (60•88) |
| RV/TLC | 0.39±0.07 |
| RV/TLC % predicted | 141±38 |
| DLCO, ml/min/mmHg | 10.55 (7.13•17.64) |
| DLCO % predicted | 42 (31•63) |
| High resolution CT findings | |
| Centrilobular nodules, n (%) | 3 (11%) |
| Ground-glass opacities, n (%) | 26 (96%) |
| Mosaic pattern, n (%) | 15 (54%) |
| Honeycombing, n (%) | 4 (14%) |
| Findings suggestive of fibrosis, n (%) | 25 (89%) |
| Emphysema, n (%) | 0 (0%) |
Data are reported as mean±standard deviation if normally distributed, or alternatively as median (25th•75th percentile).
Definition of abbreviations: HP=hypersensitivity patients; BMI=body mass index; MMRC=modified medical research council; RV=residual volume; TLC=total lung capacity; FVC=forced vital capacity; FEV1=forced expiratory volume 1s; FEF=forced expiratory flow; DLCO=diffusion of carbon monoxide.
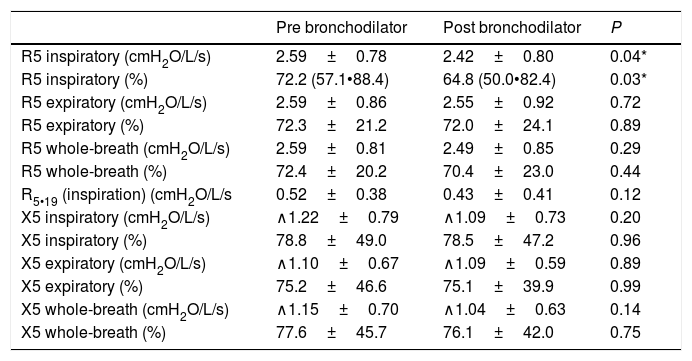
FOT results in patients with chronic HP before and after bronchodilator are shown in Table 2. Among the patients evaluated, only 3 exhibited elevated R5 (i.e., whole-breath) values. R5 on inspiration was significantly lower after bronchodilator use. Nevertheless, no patient exhibited bronchodilator response based on a fall >40% in R5 values. There was also no difference in R5•19 values after bronchodilator. Expiratory X5 was found to be significantly less negative than inspiratory X5.
Comparison of oscillometric data in patients with chronic hypersensitivity pneumonitis.
| Pre bronchodilator | Post bronchodilator | P | |
|---|---|---|---|
| R5 inspiratory (cmH2O/L/s) | 2.59±0.78 | 2.42±0.80 | 0.04* |
| R5 inspiratory (%) | 72.2 (57.1•88.4) | 64.8 (50.0•82.4) | 0.03* |
| R5 expiratory (cmH2O/L/s) | 2.59±0.86 | 2.55±0.92 | 0.72 |
| R5 expiratory (%) | 72.3±21.2 | 72.0±24.1 | 0.89 |
| R5 whole-breath (cmH2O/L/s) | 2.59±0.81 | 2.49±0.85 | 0.29 |
| R5 whole-breath (%) | 72.4±20.2 | 70.4±23.0 | 0.44 |
| R5•19 (inspiration) (cmH2O/L/s | 0.52±0.38 | 0.43±0.41 | 0.12 |
| X5 inspiratory (cmH2O/L/s) | ∧1.22±0.79 | ∧1.09±0.73 | 0.20 |
| X5 inspiratory (%) | 78.8±49.0 | 78.5±47.2 | 0.96 |
| X5 expiratory (cmH2O/L/s) | ∧1.10±0.67 | ∧1.09±0.59 | 0.89 |
| X5 expiratory (%) | 75.2±46.6 | 75.1±39.9 | 0.99 |
| X5 whole-breath (cmH2O/L/s) | ∧1.15±0.70 | ∧1.04±0.63 | 0.14 |
| X5 whole-breath (%) | 77.6±45.7 | 76.1±42.0 | 0.75 |
Data are reported as mean±standard deviation if normally distributed, or alternatively as median (25th•75th percentile). R5=resistance at 5Hz; X5=reactance at 5Hz. R5•19=resistance at 5Hz • resistance at 19Hz.
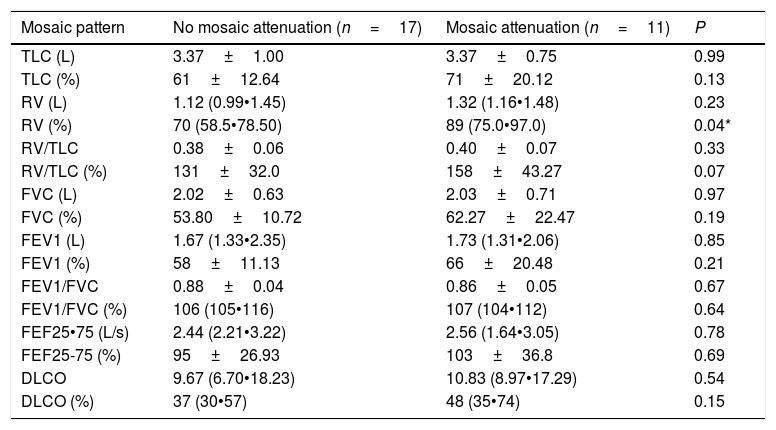
Eleven patients exhibited a significant extent of low-attenuation areas (i.e., mosaic pattern) on CT while 17 did not. There was no difference between functional data between the groups, except for higher RV values (% predicted) in patients with low-attenuation areas (Table 3).
Correlation of lung function parameters and presence or absence mosaic attenuation in chronic hypersensitivity pneumonitis.
| Mosaic pattern | No mosaic attenuation (n=17) | Mosaic attenuation (n=11) | P |
|---|---|---|---|
| TLC (L) | 3.37±1.00 | 3.37±0.75 | 0.99 |
| TLC (%) | 61±12.64 | 71±20.12 | 0.13 |
| RV (L) | 1.12 (0.99•1.45) | 1.32 (1.16•1.48) | 0.23 |
| RV (%) | 70 (58.5•78.50) | 89 (75.0•97.0) | 0.04* |
| RV/TLC | 0.38±0.06 | 0.40±0.07 | 0.33 |
| RV/TLC (%) | 131±32.0 | 158±43.27 | 0.07 |
| FVC (L) | 2.02±0.63 | 2.03±0.71 | 0.97 |
| FVC (%) | 53.80±10.72 | 62.27±22.47 | 0.19 |
| FEV1 (L) | 1.67 (1.33•2.35) | 1.73 (1.31•2.06) | 0.85 |
| FEV1 (%) | 58±11.13 | 66±20.48 | 0.21 |
| FEV1/FVC | 0.88±0.04 | 0.86±0.05 | 0.67 |
| FEV1/FVC (%) | 106 (105•116) | 107 (104•112) | 0.64 |
| FEF25•75 (L/s) | 2.44 (2.21•3.22) | 2.56 (1.64•3.05) | 0.78 |
| FEF25-75 (%) | 95±26.93 | 103±36.8 | 0.69 |
| DLCO | 9.67 (6.70•18.23) | 10.83 (8.97•17.29) | 0.54 |
| DLCO (%) | 37 (30•57) | 48 (35•74) | 0.15 |
Data are reported as mean±standard deviation if normally distributed or median (25th•75th percentile) otherwise. TLC=total lung capacity; RV=residual volume; FVC=forced expiratory capacity; FEV1=forced expiratory volume in one second; FEF25-75: forced mid-expiratory flow rate; DLCO=pulmonary diffusion of carbon monoxide.
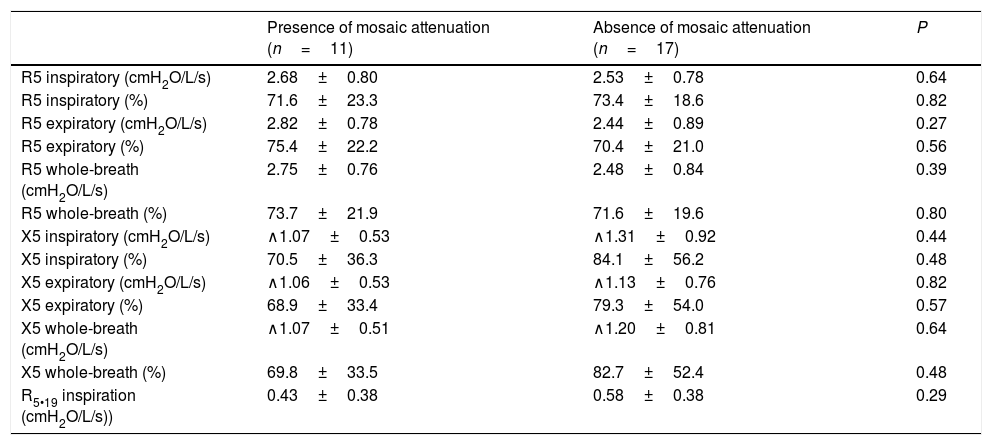
FOT data from the patients, according to the presence or absence of mosaic attenuation, are presented in Table 4. There was no statistical difference between the groups in terms of R5 or X5 values (inspiratory, expiratory, and whole-breath absolute and predicted values) between the groups.
Comparison of oscillometric data in patients with chronic hypersensitivity pneumonitis according to the presence of mosaic attenuation.
| Presence of mosaic attenuation (n=11) | Absence of mosaic attenuation (n=17) | P | |
|---|---|---|---|
| R5 inspiratory (cmH2O/L/s) | 2.68±0.80 | 2.53±0.78 | 0.64 |
| R5 inspiratory (%) | 71.6±23.3 | 73.4±18.6 | 0.82 |
| R5 expiratory (cmH2O/L/s) | 2.82±0.78 | 2.44±0.89 | 0.27 |
| R5 expiratory (%) | 75.4±22.2 | 70.4±21.0 | 0.56 |
| R5 whole-breath (cmH2O/L/s) | 2.75±0.76 | 2.48±0.84 | 0.39 |
| R5 whole-breath (%) | 73.7±21.9 | 71.6±19.6 | 0.80 |
| X5 inspiratory (cmH2O/L/s) | ∧1.07±0.53 | ∧1.31±0.92 | 0.44 |
| X5 inspiratory (%) | 70.5±36.3 | 84.1±56.2 | 0.48 |
| X5 expiratory (cmH2O/L/s) | ∧1.06±0.53 | ∧1.13±0.76 | 0.82 |
| X5 expiratory (%) | 68.9±33.4 | 79.3±54.0 | 0.57 |
| X5 whole-breath (cmH2O/L/s) | ∧1.07±0.51 | ∧1.20±0.81 | 0.64 |
| X5 whole-breath (%) | 69.8±33.5 | 82.7±52.4 | 0.48 |
| R5•19 inspiration (cmH2O/L/s)) | 0.43±0.38 | 0.58±0.38 | 0.29 |
Data are reported as mean±standard deviation if normally distributed, or alternatively as median (25th•75th percentile). R5=resistance at 5Hz; X5=reactance at 5Hz.
Our study aimed to evaluate small airway involvement in patients with chronic HP using an FOT device. The main findings of our study were as follows: all patients exhibited a restrictive pattern on PFTs; R5 was elevated in a small percentage of patients with chronic HP (8% of the entire cohort); no patient exhibited a bronchodilator response, detected as a decrease in 40% of R5 values or a significant difference in R5•19 values after bronchodilator, although R5 values on inspiration were significantly lower after salbutamol; all patients exhibited low X5 values, reflecting decreased lung compliance due to fibrosis; and, finally, R5 and X5 values were not able to discriminate patients with mosaic attenuation areas on CT.
Guerrero-Zuñiga and colleagues explored IOS measurements in chronic HP and found increased R5 and R5•20 values in 40% of patients, reflecting increased small airway resistance in this population.29 That study also reported increased AX values in this population, demonstrating lower lung compliance, which was not apparent in our study.29 Inhomogeneous ventilation, reflecting small airway disease, appeared to be best discriminated using another technique, namely ultrasonic pneumography. R5 values did not vary after treatment with prednisone and azathioprine after 4 weeks. Our results revealed a lower prevalence of increased R5 values (11%). One possible explanation for this discrepancy may be that, although FOT and IOS devices measure Rrs and Xrs at multiple frequencies, they do not necessarily yield similar values.14 Another explanation may be that the prevalence of obstructive ventilatory pattern appears to decline with longer diagnosis, reflecting progression of fibrosis.9 The median time from diagnosis in our cohort was 24 months, while Guerrero-Zuñiga evaluated patients shortly after diagnosis.29 Additionally, in accordance with our results, all patients exhibited more negative X5 values, thus reflecting decreased lung compliance.
Similar to other patients with ILD, expiratory X5 was found to be significantly less negative than inspiratory X5 in those with chronic HP.22,23 The within-breath change in X5 was a distinguishable characteristic of patients with ILD compared to those with asthma or COPD.23 Because X5 is related to lung compliance, more negative values reflect significantly reduced compliance. Moreover, because the distensibility of lung tissue is decreased in ILD patients, compliance during inspiration is likely to be more reduced compared with that during expiration due to increased elastic recoil.
Mikamo and colleagues explored FOT parameters in patients with small airway involvement detected using CT in patients with ILD.25 Absolute values of X5, Fres, and AX were significantly higher in those with small airway involvement; no difference was found in R5 values between the groups.25 In our study, we could partially reproduce these results, given that mean X5 values were higher in patients with greater extension of low-attenuation areas, although without reaching statistical significance. We hypothesised that, in addition to small airway involvement, these parameters may identify patients with less fibrosis on CT and, perhaps, higher X5 values due to more preserved lung compliance in this subgroup.
Sokai and colleagues compared rheumatoid arthritis-related pulmonary abnormalities in patients with predominant airway lesion involvement or predominant interstitial pneumonia involvement using CT scans, and found no differences in Zrs or Rrs results between these groups.38 Similarly, we did not find differences in R5 or X5 in patients with greater extension of lower-attenuation areas. Although FOT appears to be more sensitive in detecting obstruction in asthma or COPD, in ILD, results were not reproduced•this may be acknowledged as a limitation of the FOT.
Our study had some limitations that should be addressed. First, our patient cohort comprised patients with a low smoking burden. However, the exclusion of heavy smokers was intentional to avoid confounding with small airway involvement associated with smoking. Additionally, our cohort did not include patients with obstructive disease, and our results can only be extrapolated to patients with restrictive disease, which can explain the low prevalence of elevated R5 values. Nevertheless, our equipment was not able to perform at multiple frequencies like IOS. This precluded us from obtaining the resonance frequency and the reactance area (AX), which would provide additional information for evaluation of increased lung elastic recoil resulting from fibrosis, although our main focus was the evaluation of small airway involvement. Because our device did not measure data at 20Hz, we used R5•19 as a surrogate measurement for small airway resistance. Additionally, analysis of patients according to the extension of mosaic pattern areas had only a few patients included in each group, which could reduce the statistical power to detect differences between groups. Finally, studies including patients with subacute HP and chronic HP with obstructive disease are needed to evaluate small airway involvement.
In conclusion, our results suggest that FOT does not help to additionally characterise concomitant small airway involvement in patients with chronic fibrotic HP with restrictive ventilatory pattern on conventional PFTs. Nevertheless, FOT appeared to better characterise decreased lung compliance due to fibrosis reflected by X5 values. Bronchodilator therapy does not appear to induce an acute response in chronic HP patients with restrictive disease. The precise role of FOT in subacute and obstructive chronic HP, therefore, remains to be evaluated.
FundingWe acknowledge the support of the European Respiratory Society. Short-Term Research Fellowship 2015 - number 9666.
Conflict of interestsDr Ribeiro Carvalho reports personal fees from Boehringer Ingelheim, outside the submitted work. Dr Dellaca tm) reports grants from Chiesi Pharma, grants from Acutronic SA, grants from Phillips healthcare, outside the submitted work; and Raffaele Dellaca tm) was one of the founders and still own shares of a university spinoff company (RESTECH s.rl.l) that manufactures FOT based device for clinical and home based assessment of lung function. All other authors have nothing to disclose.
The authors thank colleagues from the Pulmonary Function and Exercise Physiology Laboratory and from the Interstitial Lung Disease outpatient clinic (Pulmonary Division, Heart Institute [InCor], University of Sao Paulo Medical School, Sao Paulo, Brazil) for their collaboration in this study.