Since the outcomes of lung transplants are still poorer than those obtained with others, such as heart, kidney or liver transplants, the challenge for medicine remains focused on prolonging functional graft survival. The procedure triggers significant post-surgical physiopathological changes in the lung parenchyma, the rib cage, the airways and pulmonary circulation. The patient is exposed to risks that must be identified and controlled, such as complications fully or partially attributable to immunosuppressive treatment, including cardiovascular disease, tumours and infections and, of course, chronic graft dysfunction.
The patient's prognosis will depend largely on the degree of efficacy in the prevention, early diagnosis and appropriate treatment of possible complications. Accordingly, regardless of how long it is since the transplantation, graft recipients undergo close functional and clinical monitoring. In this article, we will review the functional changes that characterise a lung transplant recipient and the usefulness of the various diagnostic techniques for patient follow-up.
Debido a que los resultados del trasplante pulmonar son todavía inferiores a los obtenidos en otros trasplantes como el cardiaco, el renal o el hepático, los retos de la medicina se deben centrar en aumentar la supervivencia funcional de los injertos. La intervención provoca marcados cambios fisiopatológicos posquirúrgicos a nivel del parénquima pulmonar, la caja torácica, las vías aéreas y la circulación pulmonar. No obstante, el paciente está sometido a riesgos que es importante conocer y controlar, como las complicaciones atribuibles total o parcialmente al tratamiento inmunosupresor, entre ellas la patología cardiovascular, la aparición de tumores, infecciones y, por supuesto, la disfunción crónica del injerto.
El pronóstico del paciente va a depender, en gran medida, de la mayor o menor eficacia en prevenir, diagnosticar precozmente y en su caso tratar de forma adecuada las posibles complicaciones. Por ello, independientemente del tiempo postrasplante, los receptores son sometidos a una estrecha monitorización funcional y clínica. En este artículo revisaremos las alteraciones funcionales características del receptor de un trasplante pulmonar y la utilidad en el seguimiento del paciente de las diferentes técnicas diagnósticas.
The advances of recent years mean that lung transplantation has become an essential treatment option for some irreversible lung diseases. The patient must confront the risks derived from immunosuppressive treatment and complications with the transplanted lung. Close patient monitoring allows the detection and diagnosis of foreseeable complications at an early stage when they are still easily reversible, before the clinical manifestations become fulminant and irreversible. The monitoring protocol must be tailored to each individual patient and to each specific stage, in accordance with the time since the transplant and the patient's progress.
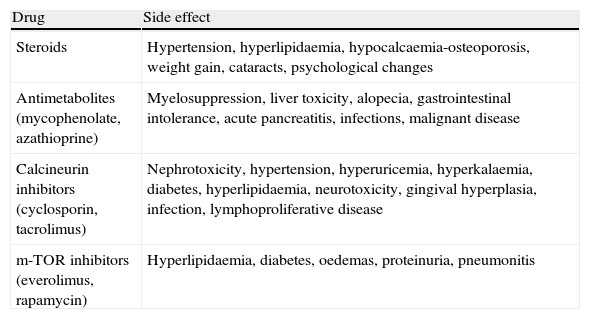
Post-transplantation monitoring aims to maintain optimal immunosuppression for each specific patient, maximising pharmacological efficacy while minimising toxicity, and to detect and control any systemic side effects1–4 (Table 1) and graft complications, such as early dysfunction, infections, tumours and acute or chronic rejection.
Principal Side Effects of Immunosuppressive Drugs.
| Drug | Side effect |
| Steroids | Hypertension, hyperlipidaemia, hypocalcaemia-osteoporosis, weight gain, cataracts, psychological changes |
| Antimetabolites (mycophenolate, azathioprine) | Myelosuppression, liver toxicity, alopecia, gastrointestinal intolerance, acute pancreatitis, infections, malignant disease |
| Calcineurin inhibitors (cyclosporin, tacrolimus) | Nephrotoxicity, hypertension, hyperuricemia, hyperkalaemia, diabetes, hyperlipidaemia, neurotoxicity, gingival hyperplasia, infection, lymphoproliferative disease |
| m-TOR inhibitors (everolimus, rapamycin) | Hyperlipidaemia, diabetes, oedemas, proteinuria, pneumonitis |
Awareness of the functional progress of the transplanted organ is indispensable for patient management. Post-surgical physiopathological changes are largely influenced by factors such as irreversible denervation of the graft and muscle involvement. The afferent and efferent nerves are severed during surgery without any evidence of subsequent re-innervation. Accordingly, even if the patient recovers practically complete lung function, tolerance to exercise is often reduced and there are changes in the response to hypercapnia, the cough reflex and mucociliary clearance. The functional progress of the transplant can be evaluated by maximum post-surgical pulmonary function, exercise tolerance, quality of life and survival.
Maximum Post-Surgical Pulmonary FunctionScarring from the trauma of surgery and the adaptation of the graft to the recipient's chest cavity affect progressive functional improvement.
Post-operative pulmonary function depends on the type of transplant, whether unilateral or bilateral, the underlying disease and post-operative complications.5
In bilateral transplant recipients, maximum post-operative pulmonary function is not affected by the underlying disease, but it is influenced by the characteristics of the graft, the recipient's chest cavity and post-operative complications. Between 6 and 9 months after surgery, FEV1 figures of between 75% and 85% and FVC from 66% to 92% may be achieved. In the first months after surgery, mild restriction is observed which normalises after the sixth month. This is due to two different mechanisms: changes in chest wall mechanics caused by the thoracotomy, and the disparity in volume between the graft and the thoracic capacity of the recipient. DLCO and gas exchange usually normalise after the third month, and exercise tolerance increases progressively as time goes by.6,7
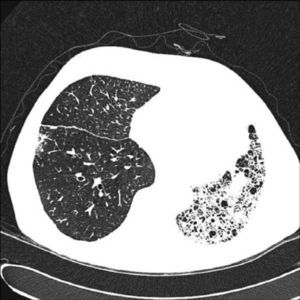
In unilateral transplant recipients, pulmonary function stabilises earlier, around the third month, because there is less trauma from the surgery. In these patients, maximum pulmonary function is lower than in bilateral transplant recipients and is also affected by the characteristics of the residual native lung. In recipients with interstitial disease, there is a tendency for the native lung to collapse, so the graft lung expands freely into the corresponding hemithorax (Fig. 1). There may be mild or moderate residual restriction and altered diffusion due to the presence of the native lung.8
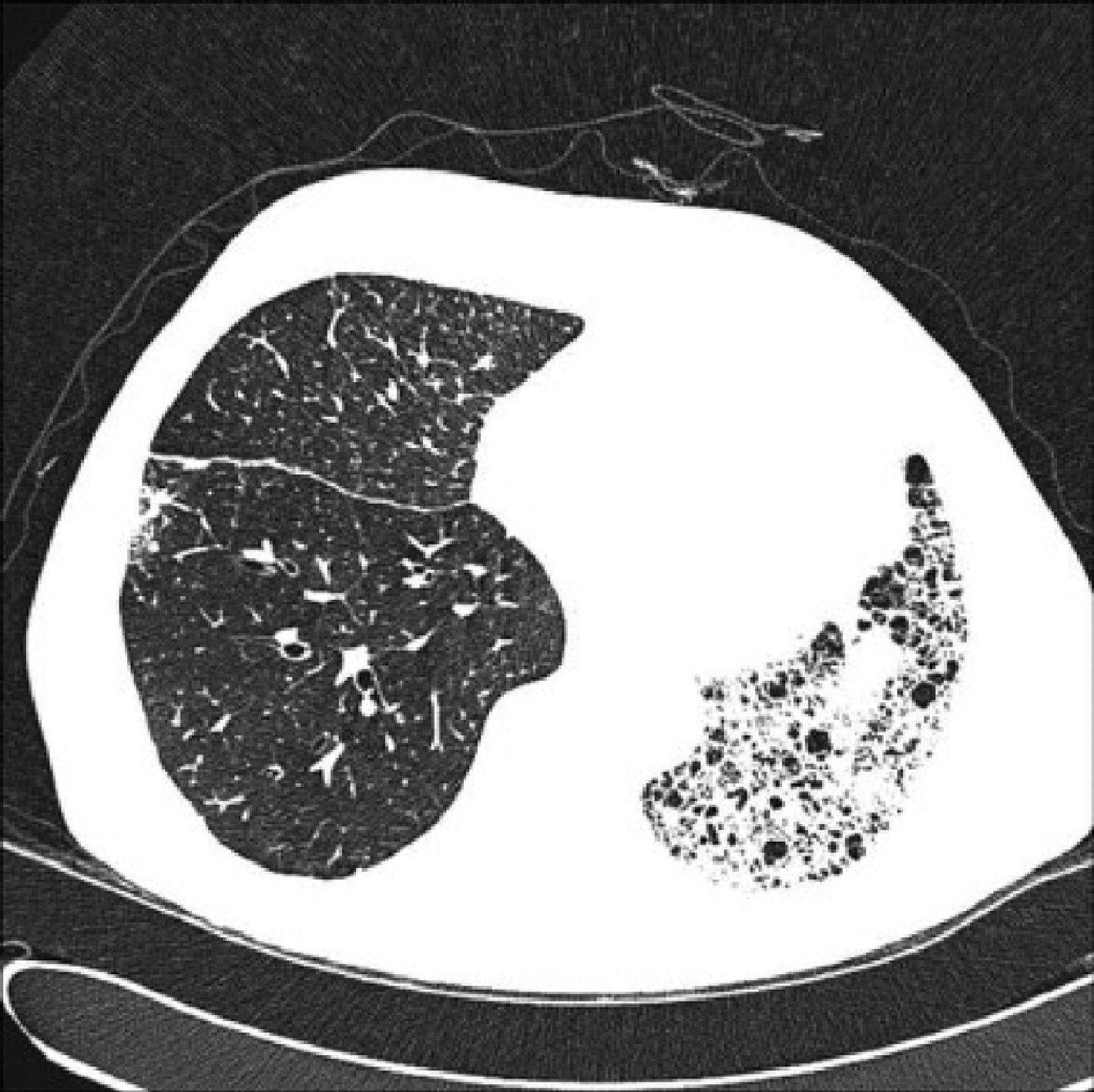
When the underlying disease is emphysema, the residual native lung tends to distend and herniate into the contralateral hemithorax, causing partial collapse of the graft (Fig. 2). Progressive air trapping produces hyperinflation in the native lung, but this does not usually present problems when the lung is functioning adequately. On occasions, the progressive distention of an emphysematous lung may compromise graft function, so volume-reducing surgery on the native lung may be necessary. Interference from an emphysematous lung may mean that functional improvement in these recipients is poorer than in those with interstitial disease. Improvement is also seen in pulmonary volumes, DLCO, gas exchange and exercise capacity.9
Exercise ToleranceAfter lung transplantation, there is a notable improvement in ventilatory capacity that often does not correlate with an increase in exercise capacity. A rapid improvement in tolerance to the 6-minute walking test occurs 3–6 months post-transplantation. However, during the effort test, lung transplant recipients show a fall in maximum oxygen consumption that is not attributable to cardiac or lung changes, anaemia or other underlying causes. Patients generally attribute their limitations during exercise to muscle fatigue. Patients are frequently malnourished before the intervention, and this, along with muscle weakness and poor physical form with loss of muscle mass, is partly responsible for limitations in exercise tolerance. A reduction in mitochondrial activity with type 2 fibre atrophy and a reduction in type 1 fibres, which may be related with chronic hypoxaemia, have been reported in some studies.10 Diminished oxidative capacity has been observed in intercostal muscle biopsies.11,12
Post-transplantation immunosuppression, particularly with calcineurin inhibitors, such as cyclosporin and tacrolimus, and corticosteroids, may contribute to muscle changes.
Quality of LifeVarious studies have shown that quality of life in transplant recipients is better than that of transplant candidates, and the improvements in quality of life are generally maintained until the development of chronic rejection.13,14
Even though transplant recipients have better general, physical and psychological health than transplant candidates, both groups, compared to the normal population, have higher levels of anxiety, self-esteem and depression. This should be taken into account in the clinical monitoring of the patient, and if there is any suspicion of psychological or psychiatric symptoms, referral should be considered.
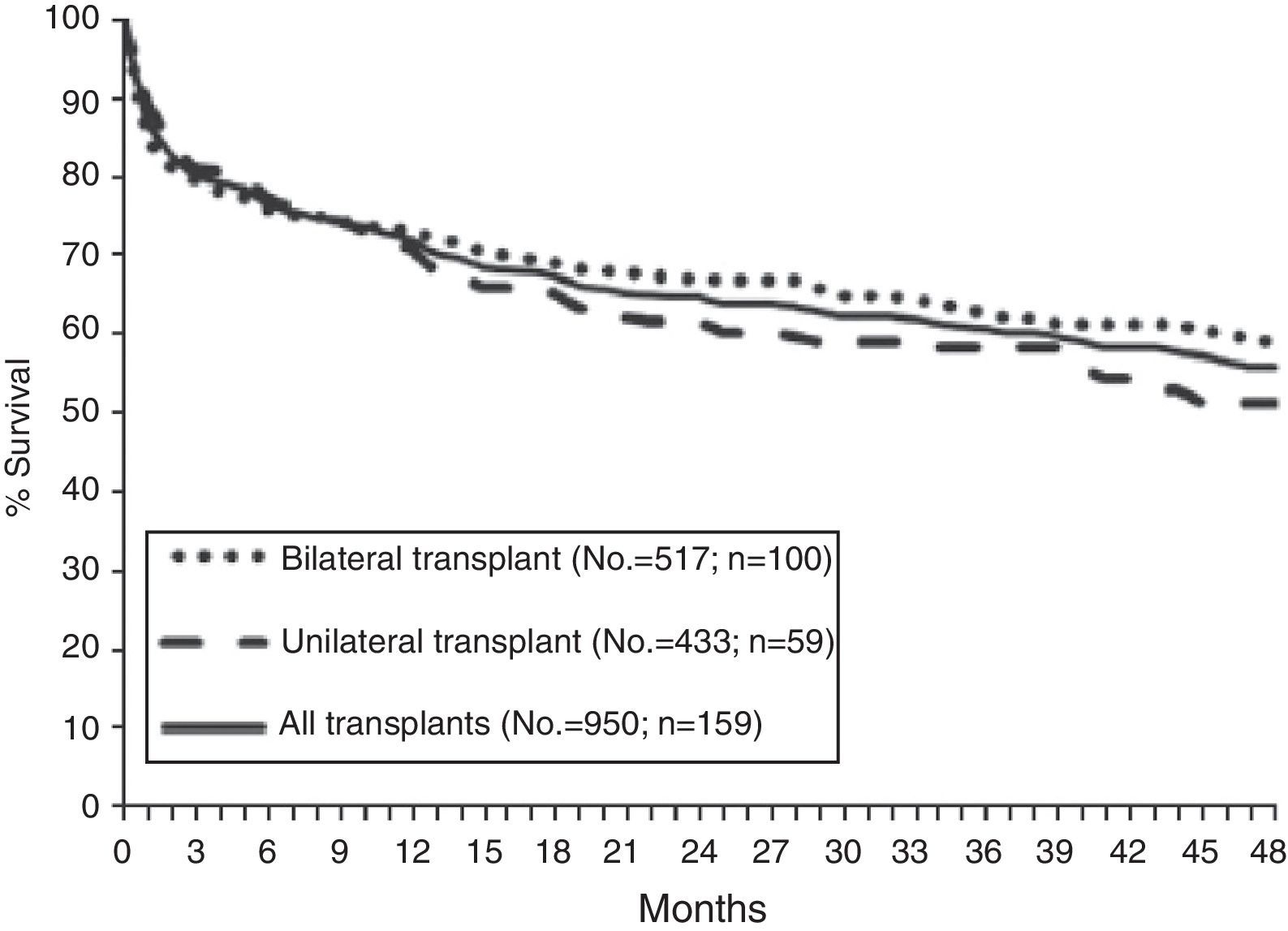
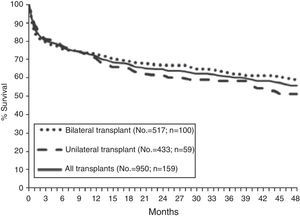
SurvivalAccording to data from the International Registry, overall survival after lung transplantation is around 79% in the first year, 64% after 3 years, 53% after 5 years and 30% after 10 years. An increase in both short-term and long-term survival has been reported over the last decade.15 These data are similar to those obtained in the Spanish Register with an unadjusted 3-month, 1-year and 3-year survival of 79, 71 and 60%, respectively (Fig. 3).16
In terms of diseases, COPD and cystic fibrosis patients have better early survival, while cystic fibrosis and pulmonary hypertension patients have better post-transplantation 10-year survival rates.15
Post-Transplantation MonitoringThe use of early, sensitive testing in monitoring the status of the graft is essential for detecting changes in functional progress. The main tools are described below.
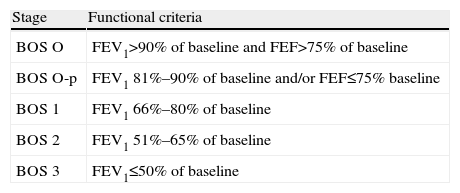
Pulmonary Function TestsForced spirometry is carried out periodically and routinely in the monitoring of lung transplant patients. Variations in maximum expired volume in the first second (FEV1) that are considered “normal” occur throughout the post-operative clinical course. This variability in spirometric values is more apparent during the first year and in unilateral graft recipients. For the correct evaluation of variations in FEV1, post-operative baseline FEV1 must be calculated, defined as the mean of the two highest values, not necessarily consecutive, obtained at least 3 weeks apart, without prior bronchodilation. Since pulmonary function improves with time after transplantation, baseline FEV1 must be periodically recalculated. A deterioration in FEV1 may be indicative of multiple problems, such as acute or chronic rejection, infection or hyperinflation of the native lung. FEV1 figures falling by more than 20% from baseline in 2 consecutive measurements obtained in a 3–6-week interval are considered a criterion for bronchiolitis obliterans syndrome (BOS), after other causes, such as infection, acute rejection or changes in bronchial anastomosis, have been ruled out.17,18 In addition, a fall in FEV1 must also be accompanied by a fall in FEV1/FVC, since a fall in FEV1 associated with a restrictive ventilatory deficit is not considered to be BOS. According to the ISHLT consensus (1993), the severity of the BOS will be weighted according to the reduction of FEV1 from baseline (Table 2). Although a fall in FEV1 remains the pulmonary function test that defines the diagnosis of BOS, small airway obstruction parameters, such FEF25%–75%, may precede a reduction of FEV1 and can be useful in the early detection of BOS.19,20 Functional parameters that indicate small airway changes have not been routinely used due to their wide intraindividual variability, particularly in unilateral transplant recipients, in whom functional variations in the residual native lung are always a confounding factor. However, some authors have observed that these parameters are highly sensitive for the early detection of BOS, even before the fall in FEV1 begins to be observed. For this reason, the BOS O-p stage, in which only FEF25%–75% is affected, was included in the classification of BOS.21
Bronchiolitis Obliterans Syndrome (BOS) Classification Stages.
| Stage | Functional criteria |
| BOS O | FEV1>90% of baseline and FEF>75% of baseline |
| BOS O-p | FEV1 81%–90% of baseline and/or FEF≤75% baseline |
| BOS 1 | FEV1 66%–80% of baseline |
| BOS 2 | FEV1 51%–65% of baseline |
| BOS 3 | FEV1≤50% of baseline |
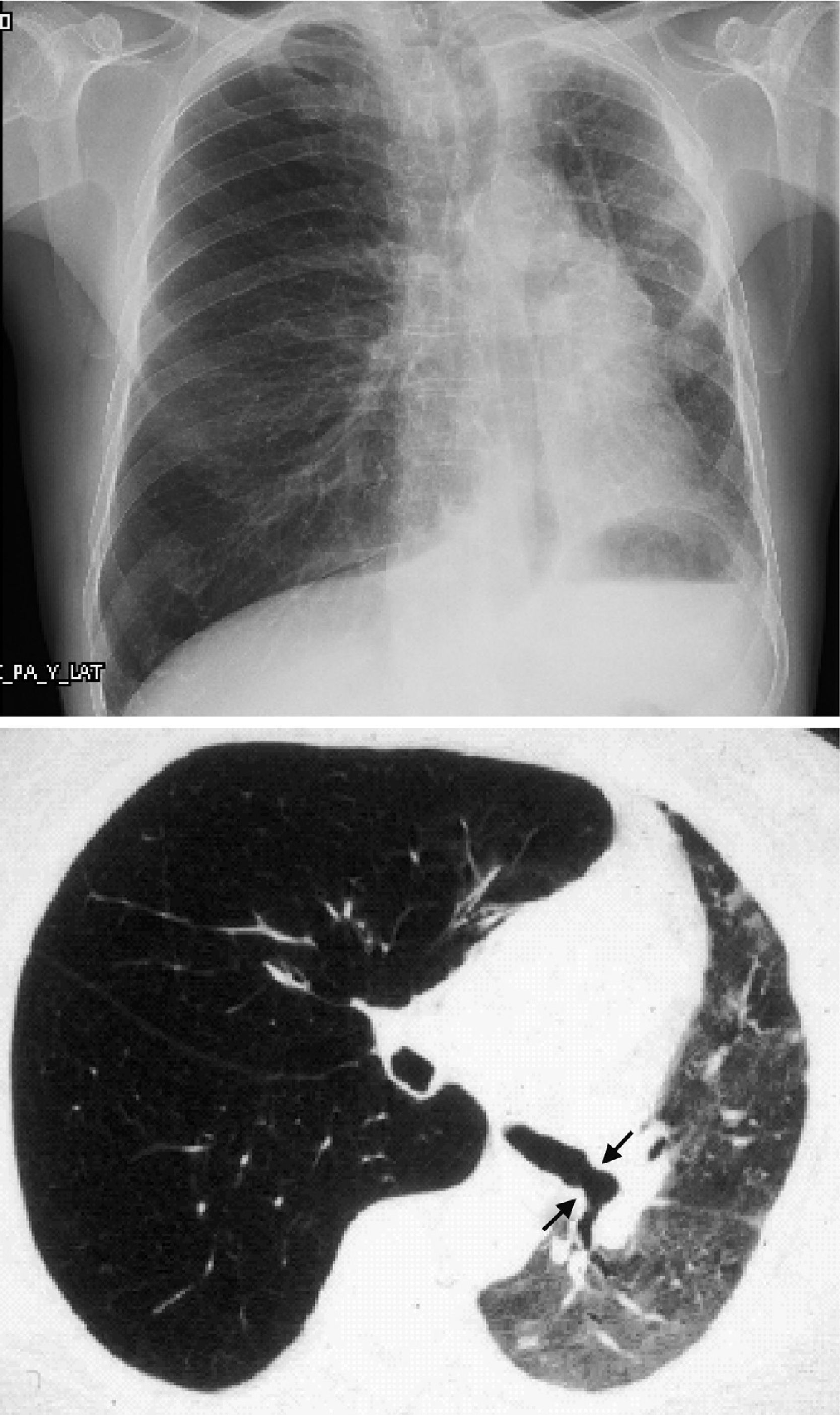
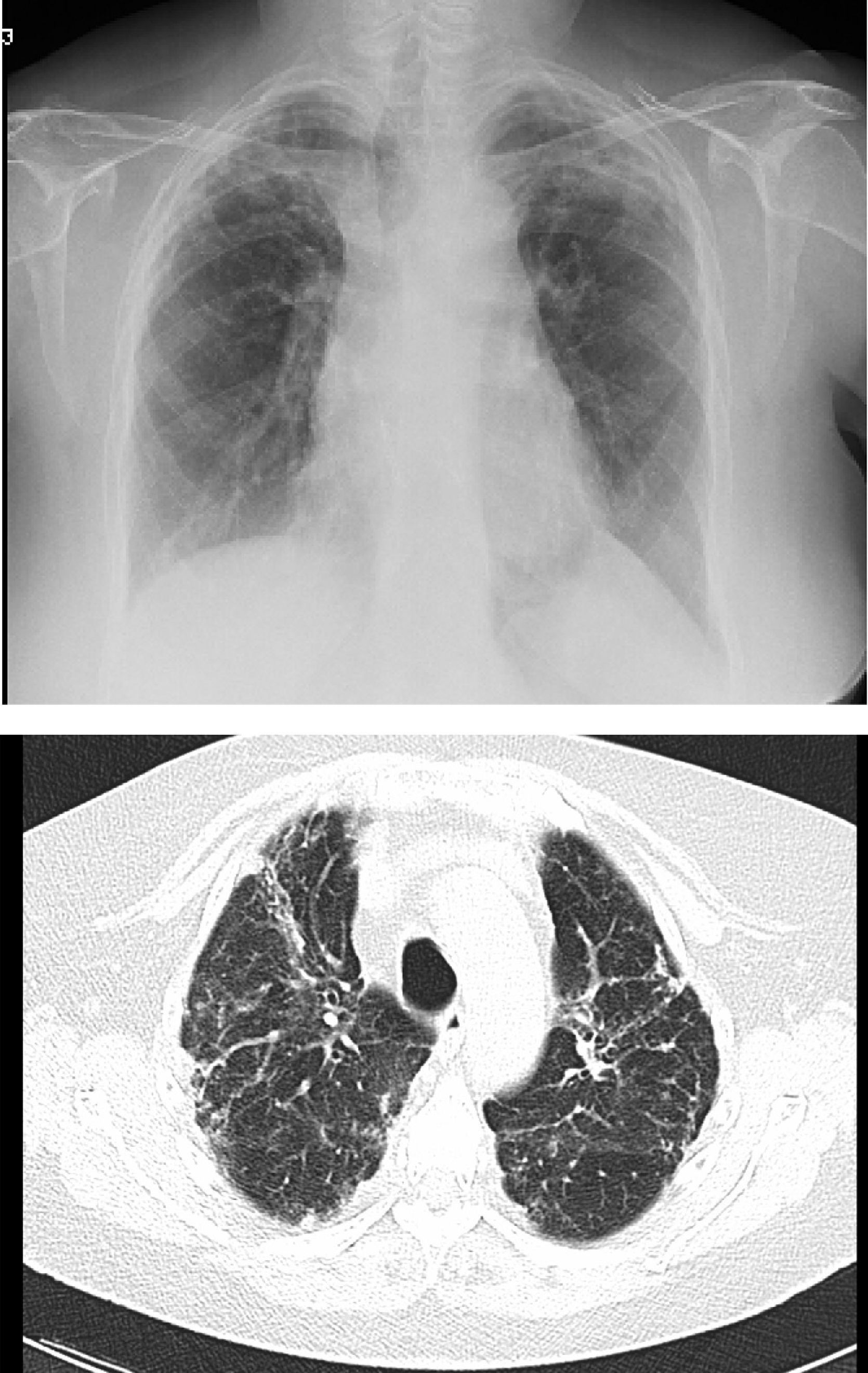
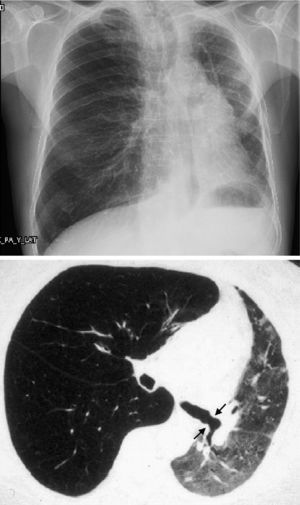
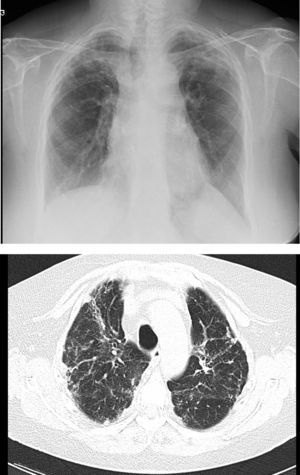
However, BOS is not the only manifestation of chronic rejection. Patterns other than obstruction that are not included under BOS have been described. Chronic lung allograft dysfunction (CLAD) is probably a better way of defining a chronic decline in FEV1 than BOS. In recent years, it has been observed that CLAD is a heterogeneous condition defining different phenotypes, including, in addition to the classic BOS, other patterns that include partial reversibility to airway obstruction, restrictive ventilatory deterioration and changes in lung parenchyma.22 Neutrophilic reversible allograft dysfunction (NRAD) is characterised by airway neutrophilia (bronchoalveolar lavage [BAL]>15% neutrophils) and functional improvement (>10% in FEV1) after azithromycin treatment.23–26 Restrictive allograft syndrome (RAS) comprises 25%–35% of CLAD and is defined as a fall from baseline in total lung capacity (TLC) greater than 10% and fibrosis, predominantly in the upper lobes (Fig. 4). It progresses more rapidly and has a poorer prognosis than BOS.27,28
Pulmonary fibrosis changes in upper lobes of a patient diagnosed with restrictive allograft syndrome (chest X-ray and chest CT).
Accordingly, full functional testing, including lung volumes, must be performed in routine post-transplant monitoring.
Home monitoring of pulmonary function with a portable spirometer is very useful for the early detection of graft complications.29 Before discharge, the patient is trained how to make three FVC and FEV1 measurements using the home spirometer in the morning and in the evening. If FEV1 values fall more than 10% from baseline, the patient must consult with the treatment centre. If the reduction in pulmonary function is confirmed on conventional spirometry, the patient must undergo a comprehensive evaluation, including chest X-ray and fibreoptic bronchoscopy, with BTB and BAL, to rule out rejection or intercurrent infection.
Exhaled Nitric OxideNitric oxide is synthesised in the cells of the respiratory tract from l-arginine, and can be measured in exhaled air (eNO). eNO measurements are reproducible in healthy individuals and eNO levels measured in the mouth correlate well with those measured in the lower respiratory tract.30,31 eNO levels do not rise in the presence of inflammatory processes of the airways, such as asthma or bronchial infections. Lung transplant patients with BOS have been seen to present raised levels of eNO and this elevation precedes in time the development of airway obstruction. Several studies of serial measurements carried out during post-transplant monitoring have reported that eNO rises occur up to 6 months before BOS is diagnosed.32 Early detection of BOS in the inflammatory stage may be useful, in that treatment can be started before irreversible fibrotic lesions of the airway develop.33
eNO increases in the initial stages of BOS when the inflammatory processes predominate over the fibrotic processes. No significant differences have been observed between unilateral and bilateral transplant recipients. In the absence of inflammatory activity, eNO levels normalise, so no differences in eNO levels are observed in patients with stable BOS, recipients without BOS and the control group.30
Although some studies have suggested that eNO is a very sensitive test for the diagnosis of BOS, its specificity is low, particularly when only one measurement is made, since other factors, such as infection, especially bacterial, can cause it to rise.
Immunological MonitoringThe ImmuKnow® technique (Cylex) measures the cell immunity status in peripheral blood.
Global immune response is quantified by measuring ATP liberated by the CD4+ lymphocytes after in vitro stimulation with phytohaemagglutinin (PHA).
Studies performed in patients with kidney and liver transplants have shown a good correlation between the development of rejection and/or infections and ImmuKnow® values above or below those observed in stable recipients.34
Low ImmuKnow® levels have been observed in lung transplant recipients with infection, compared to those of patients without infection, although no levels associated with graft rejection could be identified.35–37
The FDA recently approved ImmuKnow® as a tool for monitoring the immune status in peripheral blood of transplant recipients. However, the cut-off value indicative of a risk of rejection or infection may vary from one population to another, and only one study has been performed in lung transplant recipients.
BronchoscopyThis type of examination should preferably be performed in the transplantation centre. It can be used to diagnose complications in the bronchial suture, episodes of rejection and certain infections or colonisations that need early or preventive treatment.
Histological (transbronchial biopsy), bacteriological (BAL cultures) and cytological (cell count and proteins) studies to determine graft status can be performed using fibreoptic bronchoscopy.38–40 It can be prescribed for clinical reasons after the appearance of symptoms (cough, dyspnoea, fever) with or without a fall in spirometric parameters, or for routine graft monitoring.
Many studies41,42 have evaluated proteins and cells in BAL and their correlation with acute rejection, including elevated CD8 T cells, CD4 T cell and NK cell activation43 and raised IL-17,44 IL-1545 and gamma interferon.46 No sufficiently specific marker has been validated for the identification of patients with acute rejection and none has directly replaced the histological study of the lung tissue. Similarly, an attempt has been made in various studies to identify biomarkers in BAL for the early detection of CLAD. The predominance of neutrophils in BAL, which in some cases can be greater than 20%, has been observed in patients with a high risk of developing chronic rejection, and has helped in the identification of a subtype of chronic allograft dysfunction that responds functionally to treatment with azithromycin (NRAD). If there is no response or the patient deteriorates after treatment, BAL neutrophilia has been identified as a predictive factor for functional stability with second line treatment with photopheresis.47 Other cytokines related with the development of BOS are those associated with inflammation (IL-8, MCP-1), oxidative stress (myeloperoxidase, MPO) and extracellular matrix remodelling (metalloproteinase, MMP-2, MMP-9).48
Bronchoscopy carried out for clinical reasons has a high diagnostic yield (75%–95% for acute rejection) with low morbidity and mortality, if carried out in the absence of absolute contraindications.49 In the absence of symptoms, routine graft surveillance with fibreoptic bronchoscopy is currently under discussion, since, while it allows for the diagnosis of asymptomatic rejection episodes, it does not appear to modify patient survival. Valentine et al.50 showed that 3-year survival in patients with clinically indicated bronchoscopies was comparable to that in those who underwent routine bronchoscopy. Furthermore, recent experiences51,52 have shown wide interobserver variability in the interpretation of transbronchial biopsy by pathologists, concluding that pre-test clinical indications and functional progress are essential in the diagnosis of acute rejection.
Imaging TestsChest X-ray is routinely performed in the early post-transplant phase, every 3–6 months in the late phase and any time that it is clinically indicated. A perfusion deficit observed in the transplanted lung on quantitative lung perfusion scintigraphy would suggest stenosis of the pulmonary artery suture. CT during the first month after surgery detects changes in vascular anastomoses and/or in the pulmonary parenchyma. Subsequently, it no longer appears to offer an advantage in the early diagnosis of bronchiolitis obliterans, so it should be indicated only if the clinical symptoms or pulmonary function tests suggest some change not visible on chest X-ray.53,54
ConclusionLung transplantation is a complex and costly procedure that involves a large number of professionals before, during and after surgery. The results are not as good as might be expected, but currently it is the only therapeutic option for improving the survival and quality of life of some patients with end-stage pulmonary disease.
The success of lung transplantation depends on careful monitoring of the patient and the graft, aimed at identifying problems at an early stage, when the process may be effectively treated. This is of particular importance in bronchiolitis obliterans, which is the main factor affecting mid- and long-term lung transplantation outcomes. Current monitoring methods, such as spirometry and fibreoptic bronchoscopy, are quite specific, but not very sensitive, so we can hope that new more sensitive and more specific non-invasive monitoring tools will be developed in the future.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Laporta Hernández R, Lázaro Carrasco MT, Varela de Ugarte A, Ussetti Gil P. Seguimiento a largo plazo del paciente trasplantado pulmonar. Arch Bronconeumol. 2014;50:67–72.