The pandemic coronavirus disease 19 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been spread rapidly worldwide with considerable morbidity and mortality. COVID-19 patients have various clinical presentations: asymptomatic, exhibit mild flu-like symptoms, be severely ill or death.1,2 In addition to elder age and comorbidities, higher levels of D-dimer and C-reactive protein (CRP) and lower levels of lymphocyte and eosinophil as well as a cytokine storm are associated with disease severity in COVID-19 patients.3–6 The virus load may be a main determinant underlying the pathological diversity in COVID-19 patients.1,2,6 Thus, an effective antiviral treatment is essential to improve the prognosis of patients with COVID-19.7 In the absence of specific anti-SARS-CoV-2 agents, various drugs with antiviral potential are now used to contain the virus in COVID-19 patients. Ivermectin, a US FDA-approved anthelminthic, has garnered enormous interest for treating COVID-19 as it is safe and cheap and has strong antiviral activities against board ranges of viruses including SARS-CoV-2 in vitro.8–10 Despite the widespread use of ivermectin, to our knowledge, there is currently no published clinical reports of ivermectin in COVID-19 patients. Here, we assessed the clinical efficacy of ivermectin in COVID-19 patients.
This retrospective study enrolled a total of 325 consecutive patients with SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR) of nasal swabs in SK hospital, a unit dedicated to COVID-19 at Mymensingh Medical College Hospital (MMCH), Mymensingh, Bangladesh, from April to June 2020. Of these, the present study included 248 adult COVID-19 patients free from any other serious pathological conditions: 115 received ivermectin plus standard care (SC), while 133 received only SC. Remaining 77 patients who were under 18 years of age or transferred from other facilities and received different management approaches including partial hospital stays or treated with different therapeutic agents prior to hospital admission were excluded from the analysis. The two groups were compared in terms of time to SARS-CoV-2 negativity, disease progression (develop pneumonia to severe respiratory distress), duration of hospital stays, and mortality rate. Ivermectin was given once at dose of 12mg within 24-h after hospital admission. SC was provided as required and included antipyretics for fever, anti-histamines for cough, and antibiotics to control secondary infection. The study was approved by MMCH and informed consent was obtained from all patients or their relatives before starting treatment. Categorical variables are shown as frequencies and percentages and continuous variables as the median and interquartile range (IQR). Differences with 95% confidence intervals (CI) were computed to show the level of certainty. Paired t-tests or Pearson Chi-square test were used to analyze statistical differences. All calculations were performed using SAS, version 9.4 (Cary, NC, USA).
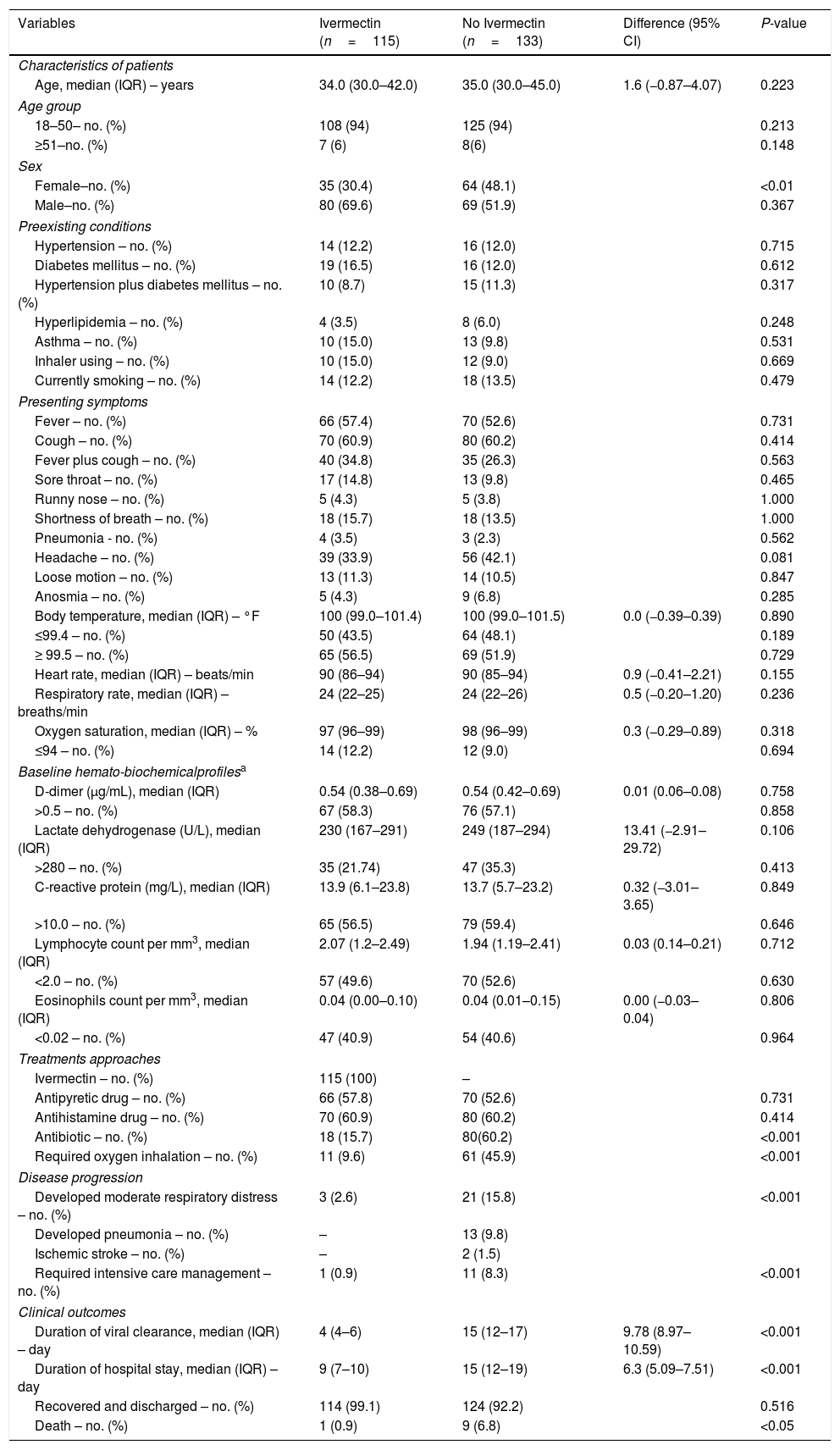
The median age of the patients was 35 (IQR 30–43) years and 60% were men. Table 1 summarizes the demographics and clinical presentations of the two groups. At the time of admission, all patients had comparatively mild/moderate disease with cough, headache, sore throat, anosmia, breathing difficulty, and pneumonia in different proportions. Some patients in both groups had underlying comorbidities like hypertension, diabetes mellitus, asthma, and hyperlipidemia. About 13% of the patients were current smokers. Also, about 58% had higher d-dimer and CRP levels, 51% had lymphopenia, and 47% had eosinopenia. However, at the time of admission, there were no significant differences in the patient profiles, presenting symptoms, comorbidities and hemato-biochemical parameters of the two treatment groups (Table 1).
Profiles, treatment approaches, clinical developments and final outcomes of SARS-CoV-2-positive patients receiving or not receiving ivermectin.*
| Variables | Ivermectin (n=115) | No Ivermectin (n=133) | Difference (95% CI) | P-value |
|---|---|---|---|---|
| Characteristics of patients | ||||
| Age, median (IQR) – years | 34.0 (30.0–42.0) | 35.0 (30.0–45.0) | 1.6 (−0.87–4.07) | 0.223 |
| Age group | ||||
| 18–50– no. (%) | 108 (94) | 125 (94) | 0.213 | |
| ≥51–no. (%) | 7 (6) | 8(6) | 0.148 | |
| Sex | ||||
| Female–no. (%) | 35 (30.4) | 64 (48.1) | <0.01 | |
| Male–no. (%) | 80 (69.6) | 69 (51.9) | 0.367 | |
| Preexisting conditions | ||||
| Hypertension – no. (%) | 14 (12.2) | 16 (12.0) | 0.715 | |
| Diabetes mellitus – no. (%) | 19 (16.5) | 16 (12.0) | 0.612 | |
| Hypertension plus diabetes mellitus – no. (%) | 10 (8.7) | 15 (11.3) | 0.317 | |
| Hyperlipidemia – no. (%) | 4 (3.5) | 8 (6.0) | 0.248 | |
| Asthma – no. (%) | 10 (15.0) | 13 (9.8) | 0.531 | |
| Inhaler using – no. (%) | 10 (15.0) | 12 (9.0) | 0.669 | |
| Currently smoking – no. (%) | 14 (12.2) | 18 (13.5) | 0.479 | |
| Presenting symptoms | ||||
| Fever – no. (%) | 66 (57.4) | 70 (52.6) | 0.731 | |
| Cough – no. (%) | 70 (60.9) | 80 (60.2) | 0.414 | |
| Fever plus cough – no. (%) | 40 (34.8) | 35 (26.3) | 0.563 | |
| Sore throat – no. (%) | 17 (14.8) | 13 (9.8) | 0.465 | |
| Runny nose – no. (%) | 5 (4.3) | 5 (3.8) | 1.000 | |
| Shortness of breath – no. (%) | 18 (15.7) | 18 (13.5) | 1.000 | |
| Pneumonia - no. (%) | 4 (3.5) | 3 (2.3) | 0.562 | |
| Headache – no. (%) | 39 (33.9) | 56 (42.1) | 0.081 | |
| Loose motion – no. (%) | 13 (11.3) | 14 (10.5) | 0.847 | |
| Anosmia – no. (%) | 5 (4.3) | 9 (6.8) | 0.285 | |
| Body temperature, median (IQR) – °F | 100 (99.0–101.4) | 100 (99.0–101.5) | 0.0 (−0.39–0.39) | 0.890 |
| ≤99.4 – no. (%) | 50 (43.5) | 64 (48.1) | 0.189 | |
| ≥ 99.5 – no. (%) | 65 (56.5) | 69 (51.9) | 0.729 | |
| Heart rate, median (IQR) – beats/min | 90 (86–94) | 90 (85–94) | 0.9 (−0.41–2.21) | 0.155 |
| Respiratory rate, median (IQR) – breaths/min | 24 (22–25) | 24 (22–26) | 0.5 (−0.20–1.20) | 0.236 |
| Oxygen saturation, median (IQR) – % | 97 (96–99) | 98 (96–99) | 0.3 (−0.29–0.89) | 0.318 |
| ≤94 – no. (%) | 14 (12.2) | 12 (9.0) | 0.694 | |
| Baseline hemato-biochemicalprofilesa | ||||
| D-dimer (μg/mL), median (IQR) | 0.54 (0.38–0.69) | 0.54 (0.42–0.69) | 0.01 (0.06–0.08) | 0.758 |
| >0.5 – no. (%) | 67 (58.3) | 76 (57.1) | 0.858 | |
| Lactate dehydrogenase (U/L), median (IQR) | 230 (167–291) | 249 (187–294) | 13.41 (−2.91–29.72) | 0.106 |
| >280 – no. (%) | 35 (21.74) | 47 (35.3) | 0.413 | |
| C-reactive protein (mg/L), median (IQR) | 13.9 (6.1–23.8) | 13.7 (5.7–23.2) | 0.32 (−3.01–3.65) | 0.849 |
| >10.0 – no. (%) | 65 (56.5) | 79 (59.4) | 0.646 | |
| Lymphocyte count per mm3, median (IQR) | 2.07 (1.2–2.49) | 1.94 (1.19–2.41) | 0.03 (0.14–0.21) | 0.712 |
| <2.0 – no. (%) | 57 (49.6) | 70 (52.6) | 0.630 | |
| Eosinophils count per mm3, median (IQR) | 0.04 (0.00–0.10) | 0.04 (0.01–0.15) | 0.00 (−0.03–0.04) | 0.806 |
| <0.02 – no. (%) | 47 (40.9) | 54 (40.6) | 0.964 | |
| Treatments approaches | ||||
| Ivermectin – no. (%) | 115 (100) | – | ||
| Antipyretic drug – no. (%) | 66 (57.8) | 70 (52.6) | 0.731 | |
| Antihistamine drug – no. (%) | 70 (60.9) | 80 (60.2) | 0.414 | |
| Antibiotic – no. (%) | 18 (15.7) | 80(60.2) | <0.001 | |
| Required oxygen inhalation – no. (%) | 11 (9.6) | 61 (45.9) | <0.001 | |
| Disease progression | ||||
| Developed moderate respiratory distress – no. (%) | 3 (2.6) | 21 (15.8) | <0.001 | |
| Developed pneumonia – no. (%) | – | 13 (9.8) | ||
| Ischemic stroke – no. (%) | – | 2 (1.5) | ||
| Required intensive care management – no. (%) | 1 (0.9) | 11 (8.3) | <0.001 | |
| Clinical outcomes | ||||
| Duration of viral clearance, median (IQR) – day | 4 (4–6) | 15 (12–17) | 9.78 (8.97–10.59) | <0.001 |
| Duration of hospital stay, median (IQR) – day | 9 (7–10) | 15 (12–19) | 6.3 (5.09–7.51) | <0.001 |
| Recovered and discharged – no. (%) | 114 (99.1) | 124 (92.2) | 0.516 | |
| Death – no. (%) | 1 (0.9) | 9 (6.8) | <0.05 | |
d-Dimer normal value ≤0.05μg/mL; Lactate dehydrogenase normal range 135–280U/L; C-reactive protein normal value ≤10.0mg/L; Lymphocyte normal range 1.50–4.00×109L–1; Eosinophil normal range 0.20–0.50×109L–1. SARS-CoV-2, severe acute respiratory syndrome coronavirus; CI, confidence interval; IQR, interquartile range.
The treatment did not produce any aberrant symptoms related to ivermectin use. None of the ivermectin-treated patients showed progressive pathology, such as pneumonia or cardiovascular complications (Table 1). On the other hand, 9.8% patient developed pneumonia and 1.5% had ischemic stroke those were not received ivermectin. Significantly fewer ivermectin-treated patients required oxygen inhalation (9.6% vs. 45.9), developed respiratory distress (2.6% vs. 15.8%), or needed antibiotic treatment (15.7% vs. 60.2%) and intensive care management (0.9% vs. 8.3%). Interestingly, the patients receiving ivermectin became SARS-CoV-2 negative more quickly (median 4 vs. 15 days; 95% CI, 8.97–10.59; P<0.001). The ivermectin-treated patients also had shorter hospital stays (median 9 vs. 15 days; 95% CI, 5.09–7.51; P<0.001). Furthermore, the mortality rate was significantly lower in the ivermectin group than SC (0.9% vs. 6.8%; P<0.05; Table 1). Of the ivermectin-treated patients, 61 were randomly assigned for follow-up assessment 10 and 20 days after discharge; none of them reported any complications.
After SARS-CoV-2 infection, the disease generally progresses within 1 week of symptom onset due to uncontrolled viral replication in the upper respiratory tract,2,6,11,12 followed by immune anomalies, and a cytokine storm.2,5,6 Thus, an effective antiviral therapy capable of blocking viral replication at the earliest time after infection may prevent disease progression. This study shows that ivermectin is safe in COVID-19 patients and efficient at rapidly clearing SARS-CoV-2 from nasal swabs (median 4 days). This was much shorter than in the COVID-19 patients receiving only SC (15 days) or receiving a combination of three antiviral drugs (7–12 days13). Furthermore, in terms of developing respiratory distress leading to ICU admission and the final outcome (discharge/death), we observed a significant clinical benefit of ivermectin in COVID-19 patients. In fact, with ivermectin, we observed quick hospital discharge (median 9 days) in 114 out of 115 patients; the remaining patient arrived with advanced disease.
Ivermectin induced rapid virological clearance that we observed in this study indicating that the preclinical efficacy of the drug against SARS-CoV-29 may be mirrored in patients. Such rapid clearing of SARS-CoV-2 (median 4 days); this is much shorter than the median duration (20 days) of viral shedding in patients with COVID-19,14 indicate that ivermectin could limit the viral spreading. Collectively, the present findings suggest that ivermectin induced rapid SARS-CoV-2 clearance could reduce COVID-19 disease progression and community transmission.
Although ivermectin is an anti-parasitic, its anti-viral capacities are known in both animals and human. In fact, ivermectin has been previously shown to exert antiviral activity in vitro against various viruses including dengue fever virus, zika virus, west nile virus, Venezuelan equine encephalitis virus, influenza virus, and SARS-CoV-2.8,9 It acts at different viral protein binding sites, reducing viral replication. Although we are not sure of the mechanisms underlying the antiviral potential of ivermectin in COVID-19 patients, the blockage of the transport of viral proteins from the cytosol to the nucleus may be one mechanism. Elucidation of the mechanisms of action of ivermectin in terms of its antiviral properties and arrest of disease progression, especially the down-regulation of pneumonia, remain to be elucidated.
In conclusion, in addition to rapid SARS-CoV-2 clearance, ivermectin seems to control the course of the disease in patients with COVID-19. As of early August 2020, the numbers of COVID-19 patients are increasing worldwide; the rise is especially marked in Latin American and South Asian countries.15 Containment strategies and patient management vary by country. Appropriate rapid management of those who are asymptomatic-to-mildly/moderately symptomatic (about 90% of all patients) is essential to prevent disease progression and community spread. Therefore, given the urgent need to manage the COVID-19 patients with a safe, cheap and widely available drug, the present findings suggest that ivermectin can be considered as a first-line treatment for containing SARS-CoV-2 to prevent severe irreversible respiratory complications and community transmission. A multicenter, double-blind, drug-controlled study will strengthen our findings.
IRB approval statusHospital approval and patient consents were taken.