In Spain, up to 3.9% of patients with severe asthma have uncontrolled disease,1–3 partly due to poor adherence to self-management plans or treatment (50 %–67%),4,5 a situation that generates higher costs.6
Telemedicine has been proposed as a potentially useful tool in asthma management,7,8 although some associated difficulties could impact on its usefulness in daily clinical practice. The current Covid-19 pandemic has highlighted the need for telemonitoring of patients who, given the situation, cannot attend face-to-face consultations. This approach is convenient, avoids travel, increases healthcare efficiency, and in certain situations is the only solution for patient care.9–11
We aimed to assess the degree of acceptance and use of a mobile application designed for the follow-up of moderate-severe, uncontrolled asthmatic patients.
In 2015, in conjunction with the Department of Computer Science of the Universidad Pública de Navarra, we developed an app with two user profiles. The patient’s profile gives access to 3 different tabs:
- □
Control: recorded daily by the patient using 4 dichotomous questions about symptoms, peak flow value of that day, last reported value of the Asthma Control Test (ACT), and 2 dichotomous questions about treatment administration and the need for rescue medication.
- □
ACT: completed monthly. Contains the 5 ACT questions.
- □
Synchronization: an additional, complementary tab for updating and refreshing data, for use only in the case of connection problems.
The doctor’s profile includes another 2 tabs:
- □
Diagnosis: severity, control, spirometry, personal peak flow, comorbidities, and various questionnaire results recorded at inclusion:
- □
ACT: self-administered questionnaire to determine degree of asthma control. Consists of 5 questions stratified from 0 to 5 points: poor control is a score ≤ 19 points.
- □
Anxiety and depression: Hospital Anxiety and Depression Scale. Self-administered 14-item questionnaire with subscales of 7 items each (anxiety in odd-numbered questions and depression in even-numbered questions). The score in each subscale ranges from 0 to 21. The higher the score, the higher the anxiety and depression. For both subscales, scores above 10 would indicate a “case”, and scores above 7 would be considered a “probable case”.
- □
Degree of functional dyspnea or hyperventilation (Nijmegen questionnaire): self-administered questionnaire consisting of 16 items scored on a scale from 0 to 4. A score of ≥ 23 suggests a diagnosis of hyperventilation syndrome.
- □
Adherence to asthma treatment: Test of Adherence to Inhalers. Evaluates the degree of therapeutic adherence and identifies patterns of poor adherence (erratic, deliberate, and unconscious). A total of 54 points can be obtained; a score ≤ 45 points is considered poor adherence.
- □
Health-related quality of life: quality of life questionnaire for patients with asthma, short version (Mini-Asthma Quality of Life Questionnaire) and EuroQol-5D. In the first, an average modification of 0.5 in the score obtained in each question, each dimension, or in the overall questionnaire is equivalent to “minimally important clinical change”. Differences of about 1.0 represent moderate change and differences > 1.5 represent large changes.
- □
- □
Incidence: record of exacerbations and need for additional treatment every 3 months.
Every day, the app automatically generates a color-coded control level (green, yellow, and red), and provides personalized instructions for treatment adjustment (at the discretion of the physician). In case of inadequate control (yellow and red), the physician receives an automatic alert and can respond immediately via the app.
Between December 2015 and December 2017, we successively enrolled 34 outpatients, aged between 18 and 75 years with a proven diagnosis of uncontrolled moderate or severe asthma (according to GINA-GEMA criteria),1,2 defined as: ACT ≤ 19 in 2 evaluations performed over a 6-month period and/or ≥ 2 exacerbations during the previous year requiring systemic corticosteroids. The study was approved by the clinical research ethics committee of the hospital. Exclusion criteria were patient with no smartphone, a primary diagnosis other than asthma, and/or the existence of debilitating mental illness. Of the total number of preselected patients, only 5.7% were excluded because they did not have a smartphone. Overall, 73% were women, mean age was 54.9 years, and mean baseline ACT was 16.5 points. The socioeconomic status of patients was not specifically assessed, but residence in rural areas was considered when assessing potential problems with the app or network connectivity, although no significant differences were identified.
Thirty patients (91%) completed the follow-up period of 1 calendar year. They attended face-to-face visits in the Severe Asthma Unit every 3 months and had access to the day hospital. The 4 lost-to-follow-up cases were patients who repeatedly failed to attend the face-to-face visits. Patients with low adherence to app use were not excluded from the analysis.
We measured the degree of satisfaction by means of a specifically designed questionnaire consisting of 6 questions scored from 1 (“strongly disagree”) to 5 (“strongly agree”) points. The mean score was 25.7 out of 30 possible points at 3 months, and was almost 1 point higher (26.5) at the end of the follow-up period. The lowest score was for app/network connectivity (mean 4.07 vs. 3.9 points at 12 months).
To evaluate the use of the app, each patient’s input was summarized and the percentage was calculated from the theoretical annual value (365 inputs). Taking into account existing studies,7,12–14 65% or higher was considered good adherence. The overall mean was 70%, sustained over time (76% at 3 months and 68% at 6, 9, and 12 months).
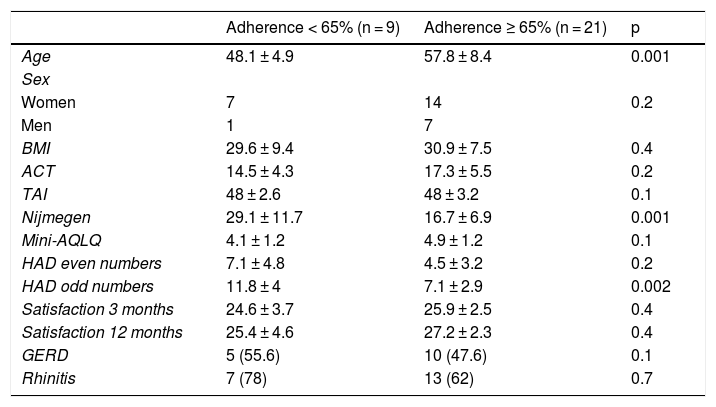
An association was revealed between adherence (<65% and ≥65%) and age, Nijmegen questionnaire, and anxiety profile on the Hospital Anxiety and Depression Scale (Table 1).
Comparison of variables between groups by app adherence.
| Adherence < 65% (n = 9) | Adherence ≥ 65% (n = 21) | p | |
|---|---|---|---|
| Age | 48.1 ± 4.9 | 57.8 ± 8.4 | 0.001 |
| Sex | |||
| Women | 7 | 14 | 0.2 |
| Men | 1 | 7 | |
| BMI | 29.6 ± 9.4 | 30.9 ± 7.5 | 0.4 |
| ACT | 14.5 ± 4.3 | 17.3 ± 5.5 | 0.2 |
| TAI | 48 ± 2.6 | 48 ± 3.2 | 0.1 |
| Nijmegen | 29.1 ± 11.7 | 16.7 ± 6.9 | 0.001 |
| Mini-AQLQ | 4.1 ± 1.2 | 4.9 ± 1.2 | 0.1 |
| HAD even numbers | 7.1 ± 4.8 | 4.5 ± 3.2 | 0.2 |
| HAD odd numbers | 11.8 ± 4 | 7.1 ± 2.9 | 0.002 |
| Satisfaction 3 months | 24.6 ± 3.7 | 25.9 ± 2.5 | 0.4 |
| Satisfaction 12 months | 25.4 ± 4.6 | 27.2 ± 2.3 | 0.4 |
| GERD | 5 (55.6) | 10 (47.6) | 0.1 |
| Rhinitis | 7 (78) | 13 (62) | 0.7 |
ACT: Asthma Control Test; BMI: body mass index; GERD: gastroesophageal reflux disease; HAD: Hospital Anxiety and Depression Scale; Mini-AQLQ: Mini Asthma Quality of Life Questionnaire; TAI: Test of Adherence to Inhalers.
The data are expressed as mean ± standard deviation or n (%).
A total of 805 alerts were generated for poor disease control, 87% of which were automatically resolved via the app itself, while remote medical intervention was necessary in 13%.
Although the number of visits to the primary care physician, to the emergency department, hospital and ICU admissions, exacerbations, and need for rescue medication were not the primary aim of this study, data from the preceding year were compared with those from the study year. We also collected data for an economic study of both direct and indirect costs, which will improve the quality of the study.
Access to the health system tailored for typical asthma variability is often difficult or impossible to achieve. Results on the effectiveness of telemedicine are inconclusive.15
Adherence to the app was reliably evaluated over a sufficiently long period, since recording was automatically performed on a daily basis, and changes in use over time could be determined (it only decreased by 8 percentage points between months 3 and 6). The number of patients lost-to-follow-up (11.7%) was acceptable and not due to problems with the app. Satisfaction improved 1 point at completion of follow-up.
We found that less adherent patients scored higher on the anxiety (Hospital Anxiety and Depression Scale) and hyperventilation (Nijmegen) questionnaires, a finding that might aid the identification of individuals who need additional support to improve their use.
Only 13% of alerts generated during the entire follow-up period required medical intervention. This means that the app can be used in daily clinical practice and public health systems without overburdening resources. In addition, it would restrict face-to-face visits to those patients who really need them, while the remaining patients could be monitored in a daily virtual follow-up, essential in situations such as those experienced in the Covid-19 pandemic.
FundingGrants and grants received: 2015 call for grants from Fundación Bancaria Caja Navarra, project no. 70504; institutional support from Teva Pharma, S.L.U., and Research Project Grant from the Basque-Navarre Society of Respiratory Pathology (SVNPAR) 2018.
Please cite this article as: Herrero Martín S, Hueto Pérez de Heredia J, Cuesta Remón A, Gómez Fernández M, Antón MM, Cabasés J. et al. ¿Es útil una aplicación móvil para los pacientes con asma moderada-grave? Arch Bronconeumol. 2021;57:556–558.