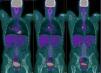
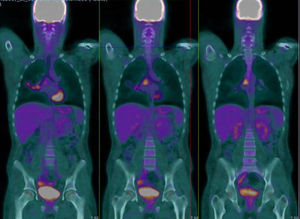
We report the case of 20-year-old man with a history of Ewing's sarcoma in the left humerus diagnosed at the age of 15, who has been in medical oncological follow-up since then. After the diagnosis, he received 5 cycles of neoadjuvant VAC/ifosfamide-VP-16, followed by radical surgery, resulting in pathological complete response according to pathology reports. The patient completed 12 adjuvant cycles of the same regimen and proceeded to regular monitoring. After 2 years free of disease, he received a diagnosis of probable pleuro-pulmonary relapse on a PET-CT, which showed hypermetabolic foci in the oblique fissure of the right lung and in the right subcarinal and hilar lymph nodes, although the latter were described as probably of a non-specific inflammatory nature, and close monitoring with imaging techniques was recommended. No known drug allergies. No toxic habits or occupational exposure. Student; no significant family history. He was referred by the medical oncology department to the respiratory medicine outpatient clinic due to the presence of mediastinal lymph nodes showing suspected tumor relapse on PET-CT. Clinical examination showed some degree of asthenia, but no other symptoms. Physical examination was normal. Clinical laboratory tests revealed completely normal serum biochemistry, complete blood count, and coagulation parameters. A computed tomography of the chest, abdomen and pelvis requested by the medical oncologist revealed a conglomerate lymph node mass in the subcarinal space, with bilateral hilar lymphadenopathies, and no other findings. Given these results, a PET-CT was requested, revealing the presence of hypermetabolic foci on the right lung and right subcarinal and pulmonary hilar lymphadenopathies, with higher metabolic intensity than the previous routine PET-CT (Fig. 1). Endobronchial ultrasound under anesthetist-controlled deep sedation was performed, revealing a rounded subcarinal lymph node mass measuring 12mm with well-limited borders, homogeneous echostructure, and absence of central hilar structure or signs of necrosis. The lesion was aspirated 3 times for in situ cytopathology in the examination room, and enough material was obtained for subsequent studies. The pathological diagnosis was metastatic Ewing's sarcoma. After diagnosis, the patient received four chemotherapy cycles with the cyclophosphamide-topotecan regimen. Tolerance was good and metabolic response was complete, and autologous peripheral blood transplantation was performed. Complete metabolic response persisted in subsequent check-ups.
Ewing's sarcoma is the second most common malignant bone disease in children. Approximately 90% of cases occur in the second decade of life, peaking at the age of 13 years. It is extremely rare in children younger than 5 years, and occurs predominantly in boys, at a ratio of 1.5–2:1. The most common clinical presentation is pain and swelling of the affected region, and a palpable mass may be present.1 Occasionally (more often in the case of metastatic disease) systemic manifestations occur, such as fever, asthenia, weight loss, leukocytosis, anemia, and raised erythrocyte sedimentation rate, which can lead to an erroneous diagnosis of acute osteomyelitis.2 The most common disease sites are the diaphysis of the long bones and the pelvis (70%–75% of cases), and it has a predilection for the scapula; it occurs more rarely in the ribs and the vertebrae, but it can affect any bone. About 15%–20% of patients also have pulmonary metastasis on diagnosis, and Ewing's sarcoma can metastasize during the disease course to bone and lymph nodes.3 Currently, standard chemotherapy for Ewing's sarcoma involves 4–6 cycles of vincristine, doxorubicin (Adriamycin) and cyclophosphamide, alternating with ifosfamide and etoposide (VDC/IE).4
Prognosis is determined by various parameters that make up the prognostic index: a poor prognosis is predicted by age >18 years, extraosseous site, size ≥8cm, distant metastasis (20%), post-therapy histology I, IIA or IIB, EWS-FLI1 type 2 translocation, and raised LDH levels. Our patient presented some features for poor prognosis, such as mediastinal metastasis confirmed on cytology by EBUS and pleuro-pulmonary metastasis shown on PET-CT. Nevertheless, he was able to benefit from the correct treatment offered by an optimal multidisciplinary therapeutic strategy.5 In the vast majority of cases, the diagnosis of Ewing's sarcoma with pulmonary involvement described in the literature is reached by transthoracic biopsy of accessible pulmonary lesions, so a successful approach to the mediastinal lesions by endobronchial ultrasound is essential. Herein lies the interest in this case: we were able to use a minimally invasive technique with high diagnostic yield to establish a differential diagnosis, ruling out other entities.
Please cite this article as: Caballero Vázquez A, García Flores P, Herrera Chilla Á. Afectación adenopática mediastínica por sarcoma de Ewing. Arch Bronconeumol. 2017;53:215–216.