We report the case of a 62-year-old man with hypertension, diabetes, and dyslipidemia, heavy former smoker and chronic alcoholic, undergoing neurological monitoring for possible Wernicke–Korsakov syndrome. In December 2015, he was diagnosed with locally advanced, high grade colorectal adenocarcinoma. After surgical intervention involving hemicolectomy, appendectomy, splenectomy, and omentectomy, the patient began FOLFOX adjuvant chemotherapy in January 2016, and received 8 cycles until April 2016, achieving stable disease on re-evaluation by computed tomography (CT). He presented in the emergency room 3 weeks after the last cycle of chemotherapy with dyspnea that had progressively worsened in the last week until it was experienced even at rest, with unproductive cough and 2 evening peaks of 38°C temperature, which did not improve after 5 days of antibiotic treatment as an outpatient. Baseline saturation on arrival was 85%. Chest radiograph confirmed the existence of infiltrates consistent with bilateral bronchopneumonia and in line with the clinical and laboratory findings: C-reactive protein (CRP) 27 (0.1–0.5) and 20000 leukocytes with left shift.
As the patient was immunocompromised (splenectomy, chemotherapy, alcoholism), wide spectrum antibiotics were started with meropenem. Urine was antigen-negative and only 1 sputum sample could be obtained, which was sterile on culture. After 10 days of treatment the patient was afebrile, but pathological signs persisted on auscultation and he was dyspneic at rest, despite oxygen therapy. The follow-up chest radiograph revealed a new pulmonary infiltrate. On the assumption that antibiotic treatment had been ineffective and given the impossibility of obtaining another sputum sample due to the patient's unproductive cough, fiberoptic bronchoscopy was performed, but no microbiological isolates could be obtained from the samples collected. After this last test, the patient peaked fever again and his dyspnea deteriorated even further. His general condition was very poor, so a third chest radiograph was performed, showing worsening with respect to the image obtained 24h previously. Treatment was intensified with vancomycin and cotrimoxazole, and the central venous access was withdrawn, in case it might have been an additional focus of infection. The patient was finally transferred to the ICU due to desaturation of around 75% despite high oxygen flow. Blood cultures obtained from the catheter and the peripheral access were both negative, as was culture of the catheter tip. The dyspnea study was completed with an echocardiogram, which was also normal. All serologies were negative, including HIV, hepatropic viruses, EBV, CMV, Mycoplasma pneumoniae and Chlamydia pneumoniae. CRP for CMV, influenza A/B Ag and galactomannan were also negative. No positive results were found in the autoimmunity study.
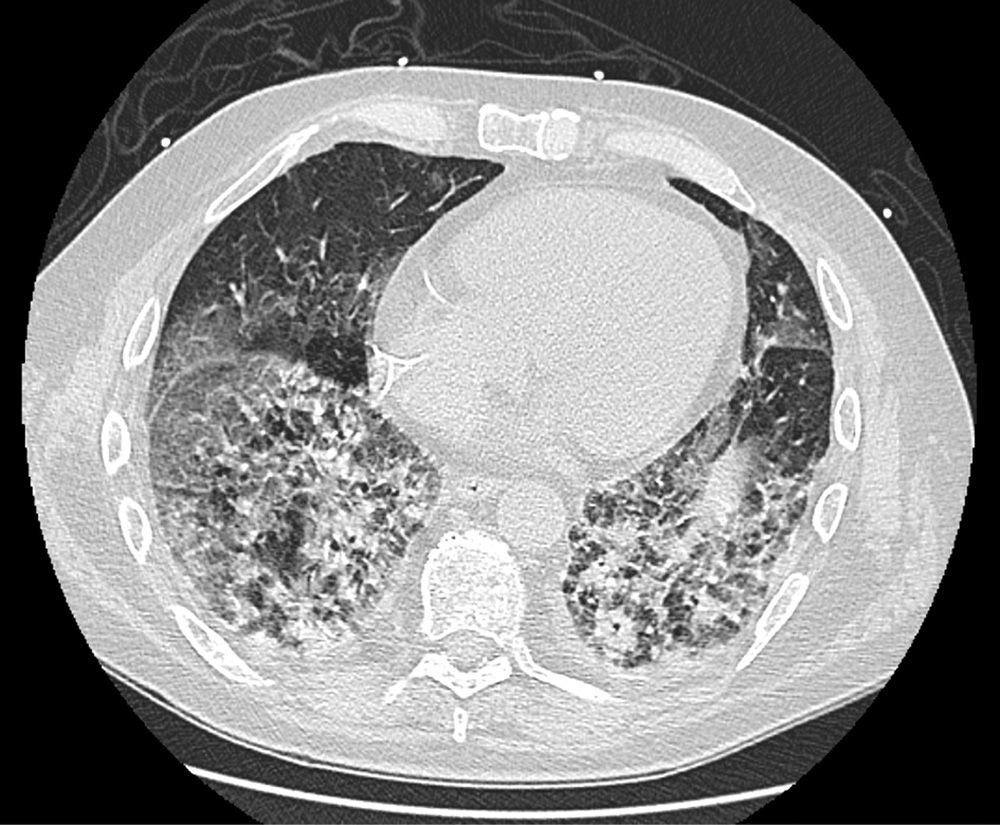
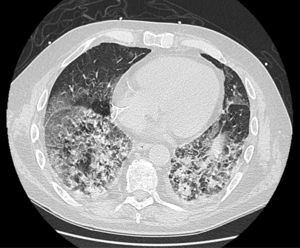
On follow-up blood tests, CRP values were seen to have plateaued at very low levels, but marked leukocytosis was observed despite the addition of antiviral and antifungal medication. Finally, a high-resolution chest CT (Fig. 1) revealed proliferative-fibrotic stage acute respiratory distress, traction bronchiectasis and bronchiolectectasis, and signs of loss of volume associated with very rapid fibrosis and extensive parenchymal involvement with areas suggestive of drug-related pneumonitis. Resistance to administered treatments, even in a final phase with high-dose corticosteroids, prompted orotracheal intubation, but even so the patient's respiratory situation deteriorated badly, and he finally died 5 weeks after admission.
Oxaliplatin is now widely used in chemotherapy regimens for colorectal cancer, and the combination of leucovorin-fluorouracil-oxaliplatin (FOLFOX) in particular has clearly shown efficacy in an adjuvant situation.1 Some anticancer agents have been closely related with lung disease. For example, bleomycin showed a high rate of mortality (The Drug-Induced Respiratory Disease Website: pneumotox), but the first studies performed to evaluate oxaliplatin toxicity did not initially show lung involvement as a side effect, and only a mild risk of hypersensitivity bronchospasm was reported. The main side effects are peripheral neuropathy and renal, hematological and gastrointestinal symptoms.2 Now, several cases have been reported associating oxaliplatin with lung damage, but as these events have generally occurred in combination regimens, it is very difficult to establish the individual contribution of each drug. At least 1 case was published on the use of single-agent oxaliplatin in adjuvancy in 2005, in which only 1 previous cycle of 5FU had been administered,3 and more recently new publications on the FOLFOX scheme have appeared.4 According to the systematic review performed in a Japanese study of 734 patients with colorectal cancer treated with FOLFOX or FOLFIRI, 11 had interstitial pulmonary disease (1.5% compared to the 0.4% reported by the FDA for complications of this type with oxaliplatin).5 Mean time to appearance was 20 days after the last cycle, with a mean of 10 FOLFOX cycles and oxaliplatin dose of 85mg/m2.
This toxicity appears to be due to glutathione depletion (an antioxidant molecule), a hypothesis that was extrapolated from a study that demonstrated liver damage with oxaliplatin caused by perivascular and endothelial involvement.6
The most common clinical symptoms are cough and dyspnea, and fever is uncommon. Radiological findings usually include interstitial infiltrate and ground glass areas. Moreover, although histology is available in very few cases, the most common findings are diffuse alveolar damage and organizing pneumonia.7 Recommended treatment consists of support and high-dose systemic corticosteroids, but despite this, mortality is high.8
In conclusion, no clear evidence of oxaliplatin-induced lung toxicity is available beyond the cases reported, but that these suggest that not only is it a possibility, but that if it occurs, it can be so severe as to cause a fatal outcome, as we ourselves have witnessed. Arriving late at a definitive diagnosis, after all other possibilities are ruled out, complicates the situation further. The pre-existence of fibrosis or any other underlying disease will aggravate the situation even further,9 so great caution and close clinical and imaging follow-up must be employed when indicating oxaliplatin in this patients.
Please cite this article as: Cendra CS, Martel IJ, Abad DG. Enfermedad pulmonar intersticial secundaria a oxaliplatino. Una complicación infrecuente pero posible. Arch Bronconeumol. 2017;53:213–215.