Severe bronchiolitis (i.e., bronchiolitis requiring hospitalization) during infancy is a heterogeneous condition associated with a high risk of developing childhood asthma. Yet, the exact mechanisms underlying the bronchiolitis-asthma link remain uncertain. Birth cohort studies have reported this association at the population level, including only small groups of patients with a history of bronchiolitis, and have attempted to identify the underlying biological mechanisms. Although this evidence has provided valuable insights, there are still unanswered questions regarding severe bronchiolitis-asthma pathogenesis. Recently, a few bronchiolitis cohort studies have attempted to answer these questions by applying unbiased analytical approaches to biological data. These cohort studies have identified novel bronchiolitis subtypes (i.e., endotypes) at high risk for asthma development, representing essential and enlightening evidence. For example, one distinct severe respiratory syncytial virus (RSV) bronchiolitis endotype is characterized by the presence of Moraxella catarrhalis and Streptococcus pneumoniae, higher levels of type I/II IFN expression, and changes in carbohydrate metabolism in nasal airway samples, and is associated with a high risk for childhood asthma development. Although these findings hold significance for the design of future studies that focus on childhood asthma prevention, they require validation. However, this scoping review puts the above findings into clinical context and emphasizes the significance of future research in this area aiming to offer new bronchiolitis treatments and contribute to asthma prevention.
Infant bronchiolitis is the most common lower respiratory infection and the leading cause of infant hospitalizations in the U.S.1 Severe bronchiolitis (i.e., bronchiolitis requiring hospitalization) is also a major risk factor for the development of childhood asthma.2,3 This bronchiolitis-asthma association is described by both birth cohort studies and bronchiolitis cohort studies.4,5 Birth cohort studies report that, at the population level, infants with a history of severe bronchiolitis are at higher risk of childhood asthma.4,6 Likewise, cohort studies of severe bronchiolitis demonstrate that approximately 30% of infants with severe bronchiolitis develop asthma by age 6–7 years.2,7 Yet, the exact mechanisms underlying the bronchiolitis-asthma link remain uncertain.
While bronchiolitis has long been considered a single disease entity,8 recent cohort studies describe clinical phenotypes of bronchiolitis that are associated with increased risk for childhood asthma.2,5,9–11 For example, infants hospitalized with rhinovirus bronchiolitis with a history of previous wheezing episodes and eczema have a higher risk of developing asthma compared to those with RSV infection.2,5,12 However, the bronchiolitis-asthma link involves a complex interplay among multi-level factors, such as respiratory viruses and non-virus exposures (i.e., air pollution, climate exposures), host genetics, and immune responses.13–15 Therefore, it is essential to gain a deeper understanding of these intricate interrelationships.
Toward this direction, recent studies have utilized unbiased and integrated (i.e., clustering) approaches in the analysis of multi-level omics data (e.g., the genome, epigenome, transcriptome, proteome, metabolome, microbiome, metatranscriptome) from nasal airway samples in addition to peripheral blood samples.16–19 These emerging findings help us understand that asthma susceptibility is not defined solely by genetic variation at a single locus (e.g., 17q21 locus)—a finding that derives from a single omics approach.20–22 A more plausible framework involves, for example, genetically driven metabolites and the genetic loci regulating these metabolites (e.g., sphingolipids with genetic variation at 1q32 locus) associated with the asthma risk, a finding that derives from an integrative genetic-metabolomic approach.18 Importantly, clustering approaches have identified distinct bronchiolitis profiles (i.e., endotypes) at differential risks for childhood asthma.23,24 Bronchiolitis endotyping is important, as it is the most suitable research approach to address disease heterogeneity.
Despite the increasing research significance, bronchiolitis endotyping approaches leave us with the challenge of clinical translation (i.e., how to use the information for the development of targeted bronchiolitis treatments or of strategies for the primary prevention of asthma). Rising to this challenge would represent the beginning of precision medicine for a large population of children. This review focuses on the description of severe bronchiolitis endotypes at differential risks for development of childhood asthma. In addition, this review summarizes evidence deriving from non-clustering approaches in birth and bronchiolitis cohort studies with the aim to define susceptibility to childhood asthma.
Literature searchThis is a scoping review discussing past and current evidence related to molecular (i.e., omics) profiles in the general population and in infants with severe bronchiolitis, examining associations with a high risk of asthma development. Relevant studies have been identified through query of the Medline, Embase, and Cochrane databases for English language articles published up to September 2023 using the terms “birth cohort”, “bronchiolitis”, “bronchiolitis cohort”, “genomics”, “epigenomics”, “transcriptomics, “proteomics”, “metabolomics”, “microbiome”, “omics”, “endotype”, and “asthma”. Studies have been selected for discussion in this review based on topic relevance. Publications cited by articles identified through the search strategy have been included as appropriate.
Evidence reviewSevere bronchiolitis in infancy and childhood asthma risk: what we know and which the knowledge gap isFor decades, bronchiolitis has been considered and managed as a single disease entity.8,25,26 However, it is recognized that severe bronchiolitis is heterogeneous in terms of both clinical presentation and the risk of chronic respiratory sequelae, such as recurrent wheeze and childhood asthma.2,27,28 Studies have identified several risk factors for subsequent asthma development by utilizing a single-level approach.29–31 For example, specific respiratory viruses (e.g., respiratory syncytial virus [RSV] or rhinovirus) have been consistently identified as risk factors for childhood asthma development.29,32–34 Despite the limited data, it is still possible to draw some insights- For example, infants with rhinovirus bronchiolitis have a 3 times higher risk of asthma compared to those with RSV bronchiolitis.35 In addition, traffic-related air pollution or the development of early-life allergic sensitization are associated with an increased risk for childhood asthma.29,36
While the identification of these single-level risk factors has advanced our understanding, they do not address the complex interplay between exposures at multiple levels (e.g., environmental exposures, host genome, transcriptome, metabolome, and microbiome) and how they may contribute to the bronchiolitis-asthma link.16,23
How omics approaches can help us address the knowledge gapOmics approaches enable the comprehensive characterization and analysis of biological molecules.37 Therefore, these approaches provide valuable insights into the structure and function of human tissues, which aid further in the identification of molecular mechanisms underlying disease development that serve as potential therapeutic targets.28,37,38 Birth and bronchiolitis cohort studies from the U.S. (e.g., the Multicenter Airway Research Collaboration [MARC], RSV bronchiolitis in Early Life [RBEL], Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure [INSPIRE], Childhood Origins of Asthma [COAST]) and Europe (e.g., Breathing Together, Copenhagen Prospective Studies on Asthma [COPSAC] and Manchester Asthma and Allergy study [MAAS]) utilize mainly non-clustering and, few of them, clustering omics approaches (i.e., endotyping) with the aim to examine the mechanisms that underlie the development of childhood asthma.17,39–44 To date, most severe bronchiolitis endotyping data derive from the MARC-35 cohort study.13,17,23,24,45 MARC-35 is severe bronchiolitis cohort that completed enrolment of 1016 hospitalized infants across 17 sites in the US. In this diverse cohort (53% African American or Hispanic), investigators collected biospecimens, including nasal swabs, nasopharyngeal aspirates, and peripheral blood samples at the index hospitalization.9 The following sections discuss available evidence from both non-clustering and clustering omics approaches in birth and bronchiolitis cohort studies.
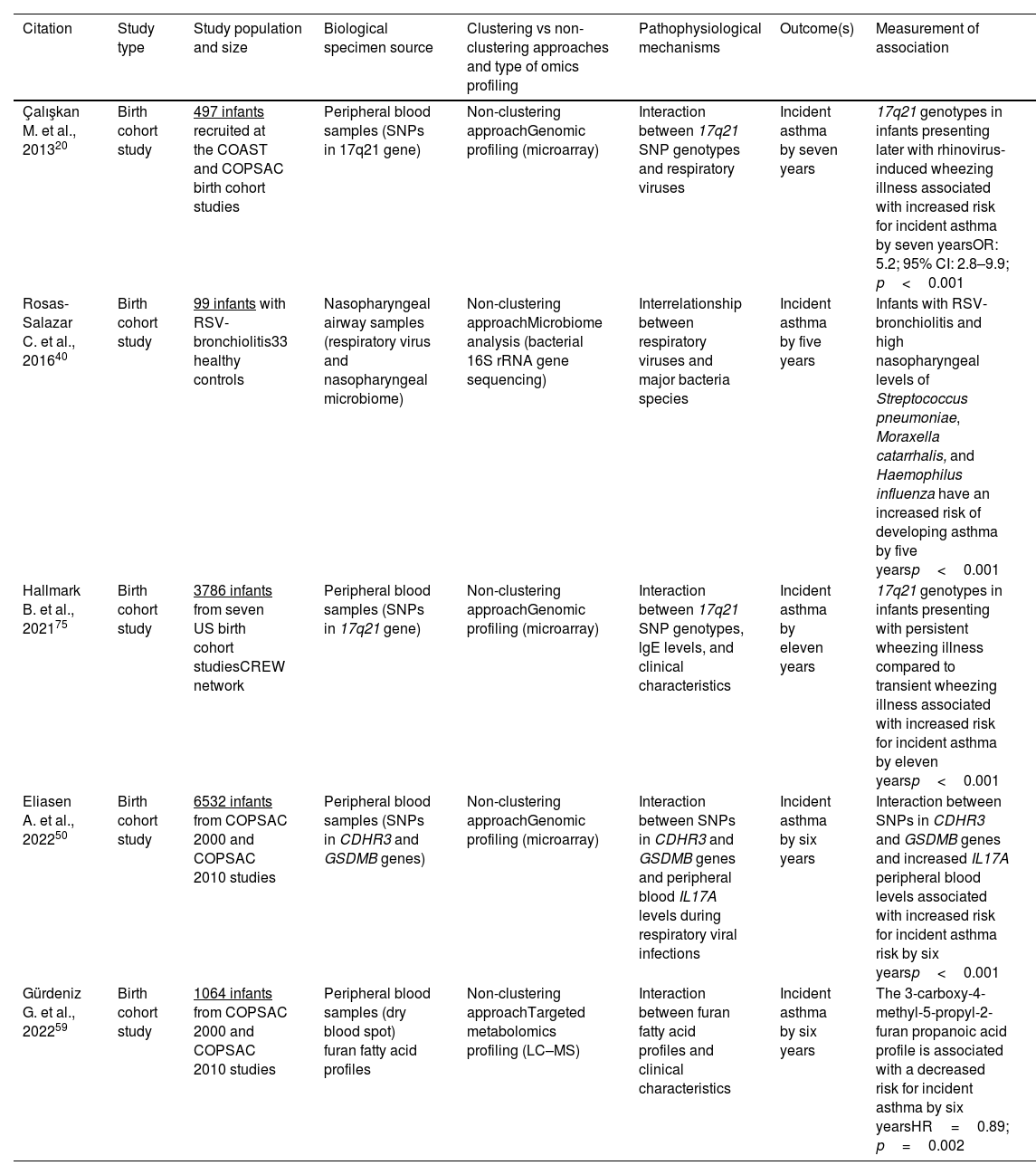
Omics approaches in birth cohort studiesU.S. and European birth cohort studies have advanced our understanding around childhood asthma etiology by using non-clustering (i.e., non-grouping) omics (e.g., genomic, epigenomic, metabolomic, microbiome) approaches. These studies do not focus solely on infants with severe bronchiolitis. Representative findings from these studies, including types of samples used and methodology (e.g., omics approach) are discussed below and are summarized in Table 1.
Representative birth cohort studies describing associations of omics non-clustering approaches and endotypes with the development of childhood asthma.
| Citation | Study type | Study population and size | Biological specimen source | Clustering vs non-clustering approaches and type of omics profiling | Pathophysiological mechanisms | Outcome(s) | Measurement of association |
|---|---|---|---|---|---|---|---|
| Çalışkan M. et al., 201320 | Birth cohort study | 497 infants recruited at the COAST and COPSAC birth cohort studies | Peripheral blood samples (SNPs in 17q21 gene) | Non-clustering approachGenomic profiling (microarray) | Interaction between 17q21 SNP genotypes and respiratory viruses | Incident asthma by seven years | 17q21 genotypes in infants presenting later with rhinovirus-induced wheezing illness associated with increased risk for incident asthma by seven yearsOR: 5.2; 95% CI: 2.8–9.9; p<0.001 |
| Rosas-Salazar C. et al., 201640 | Birth cohort study | 99 infants with RSV-bronchiolitis33 healthy controls | Nasopharyngeal airway samples (respiratory virus and nasopharyngeal microbiome) | Non-clustering approachMicrobiome analysis (bacterial 16S rRNA gene sequencing) | Interrelationship between respiratory viruses and major bacteria species | Incident asthma by five years | Infants with RSV-bronchiolitis and high nasopharyngeal levels of Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus influenza have an increased risk of developing asthma by five yearsp<0.001 |
| Hallmark B. et al., 202175 | Birth cohort study | 3786 infants from seven US birth cohort studiesCREW network | Peripheral blood samples (SNPs in 17q21 gene) | Non-clustering approachGenomic profiling (microarray) | Interaction between 17q21 SNP genotypes, lgE levels, and clinical characteristics | Incident asthma by eleven years | 17q21 genotypes in infants presenting with persistent wheezing illness compared to transient wheezing illness associated with increased risk for incident asthma by eleven yearsp<0.001 |
| Eliasen A. et al., 202250 | Birth cohort study | 6532 infants from COPSAC 2000 and COPSAC 2010 studies | Peripheral blood samples (SNPs in CDHR3 and GSDMB genes) | Non-clustering approachGenomic profiling (microarray) | Interaction between SNPs in CDHR3 and GSDMB genes and peripheral blood IL17A levels during respiratory viral infections | Incident asthma by six years | Interaction between SNPs in CDHR3 and GSDMB genes and increased IL17A peripheral blood levels associated with increased risk for incident asthma risk by six yearsp<0.001 |
| Gürdeniz G. et al., 202259 | Birth cohort study | 1064 infants from COPSAC 2000 and COPSAC 2010 studies | Peripheral blood samples (dry blood spot) furan fatty acid profiles | Non-clustering approachTargeted metabolomics profiling (LC–MS) | Interaction between furan fatty acid profiles and clinical characteristics | Incident asthma by six years | The 3-carboxy-4-methyl-5-propyl-2-furan propanoic acid profile is associated with a decreased risk for incident asthma by six yearsHR=0.89; p=0.002 |
Of genetic variants associated with the asthma risk, genome-wide association studies (GWAS) and candidate-gene association studies link those in orosomucoid-like sphingolipid biosynthesis regulator 3 (ORMDL3) in chromosome 17q21 to development of childhood asthma.46,47 Other commonly reported loci in the association with asthma include, for example, the Denn domain containing 1B (DENND1B), interleukin (IL) 1 receptor ligand 1(IL1RL1), RAD50, cadherin-related family member 3 (CDHR3), and IL33 loci.48 These loci are also associated with a higher risk for a childhood asthma phenotype characterized by a high number of virus-induced exacerbations.49 Additionally, a Danish GWAS reported an interaction between CDHR3 and gasdermin B (GSDMB) loci in the development of early childhood asthma.50 Taken together, these findings, albeit not deriving from infants with severe bronchiolitis, demonstrate the importance of understanding genetic heterogeneity in childhood asthma and its phenotypes.51
Epigenomic approachesWe next summarize epigenomics evidence from non-clustering approaches. A consortium-based meta-analysis of epigenome-wide association studies (EWAS) utilizing whole blood samples and identified hypomethylated 5′-C-phosphate-G-3′ (CpG) sites that are associated with high asthma risk.52 These CpG sites and the associated transcriptional profiles indicate, for example, activation of eosinophils and reduction in naïve T cells as possible important mediators in the pathway linking environmental exposures at early years to childhood asthma development.52 Data from other EWAS using whole blood samples also report an epigenetically driven eosinophil activation pattern in the development of childhood asthma.53,54
In addition to the identification of individual CpGs, a recent EWAS focuses on differentially methylated regions (DMRs) to reduce the dimension of epigenome-wide methylation data.54,55 In this context, an epigenome-wide meta-analysis of associations between whole blood methylation sites and risk of school-age asthma identified 36 DMRs correlated with expression of genes that are already targeted in asthma treatments (e.g., IL5R gene, potassium voltage-gated channel subfamily H member [KCNH] gene).54 The substantial impact of this research would be the use of CpGs and DMRs identified in birth cohort studies as potential therapeutic targets or biomarkers for subsequent asthma risk.56,57
Metabolomic approachesIn addition to epigenomic profiles, an increasing number of scientific reports describe that the profiling of end products of cellular metabolism (i.e., metabolomics) is linked to the development of persistent wheezing and asthma in childhood.58–60 For example, recent findings from the COPSAC cohort using dried blood spots at birth demonstrate peripheral blood metabolomic profiles at higher risk for asthma development.59 Specifically, decreased newborn tryptophan, bile acid, and phenylalanine metabolism were associated with a higher asthma risk.59 In support of this evidence, bile metabolism pathways detected in plasma samples were also implicated in asthma pathogenesis following RSV bronchiolitis in U.S. birth cohort studies.61 Furthermore, the COPSAC2010 study has investigated whether a dietary intervention during pregnancy can decrease the risk of asthma.60 Indeed, a randomized controlled trial of fish oil-derived n-3 long-chain polyunsaturated fatty acid supplements to pregnant mothers found that the intervention reduces the risk of incident asthma by age 5 years.60 The study also found that infants born to mothers who received fish oil supplements and had reduced tryptophan-related metabolites and increased tyrosine- and glutamic acid-related metabolites were less likely to develop asthma by age 5 years.60 In summary, birth cohort studies have identified that metabolome regulation in early life can be associated with risk of childhood asthma.
Microbiome approachesIn addition to metabolomic profiles, changes in the airway microbiome are considered to play a key role in the development of childhood asthma.62,63 These data derive from analysis of upper airway specimens since lower airway sampling (e.g., bronchoscopy) is invasive in young infants and usually takes place under absolute indications (e.g., intubation). In addition, studies have suggested that upper airway sampling possibly represents the lung transcriptome and microbiome profiles in children.64,65
Firstly, findings from the COPSAC2010 cohort reported that reduced microbiome alpha diversity and an increase in relative abundance of Veillonella and Prevotella in the upper airway at age 1 month was associated with higher asthma development risk by age 6 years.66 A higher relative abundance of these genera is also linked to upper airway immune profiles characterized by reduced levels of proinflammatory mediators (i.e., tumor necrosis factor-α [TNF-α] and IL-1β).66 In addition to these microbiome profiles, a U.S. birth cohort study also reported that detection of Streptococcus pneumoniae and Moraxella catarrhalis at the nasopharynx of infants with reported RSV-positive bronchiolitis was associated with increased asthma risk by age 6 years.40
Furthermore, COPSAC cohort studies have identified that several factors are critical in determining the diversity of the upper airway and gut microbiome of children, and their association with asthma risk. These factors include the mode of delivery, the presence of older siblings, and the age gap to the closest older sibling.67,68 The above findings showcase that changes in airway microbiome in early life may be linked to the development of childhood asthma.
On the whole, birth cohort studies have enhanced our understanding around early-life origins of childhood asthma, despite not focusing on infants at high risk to develop childhood asthma (i.e., infants with severe bronchiolitis). The next section focuses on evidence from severe bronchiolitis cohort studies.
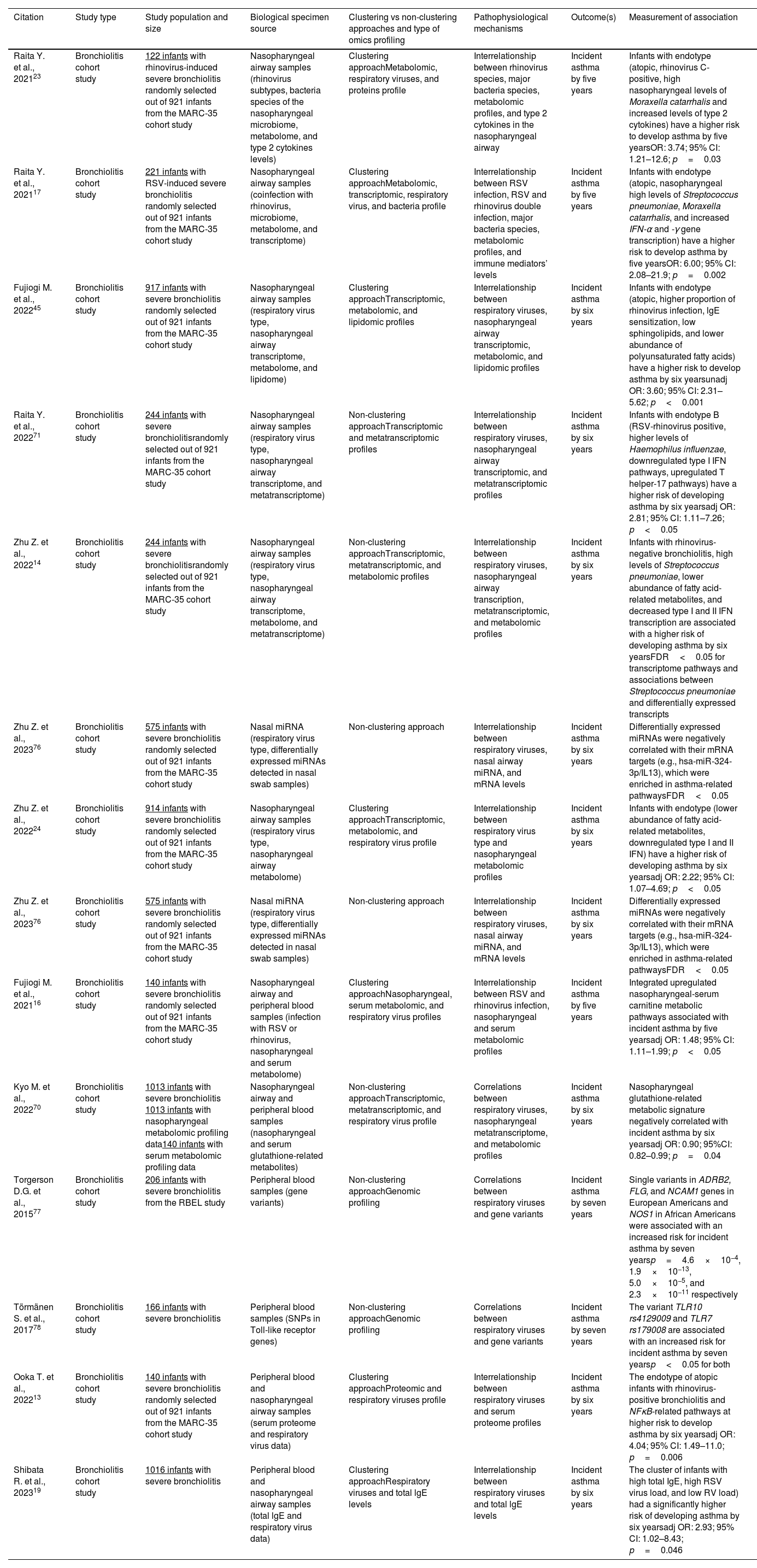
Omics approaches in severe bronchiolitis cohort studiesReported omics data from both MARC-35 and other severe bronchiolitis cohorts69 derive from both non-clustering (i.e., associations between severe bronchiolitis omics profiles and asthma development)14,18,70,71 and clustering (endotyping)13,17,23,24,45,72 approaches. Therefore, in this section and in Table 2, we summarize representative findings from both approaches.
Representative bronchiolitis cohort studies describing associations between omics endotypes and the development of childhood asthma.
| Citation | Study type | Study population and size | Biological specimen source | Clustering vs non-clustering approaches and type of omics profiling | Pathophysiological mechanisms | Outcome(s) | Measurement of association |
|---|---|---|---|---|---|---|---|
| Raita Y. et al., 202123 | Bronchiolitis cohort study | 122 infants with rhinovirus-induced severe bronchiolitis randomly selected out of 921 infants from the MARC-35 cohort study | Nasopharyngeal airway samples (rhinovirus subtypes, bacteria species of the nasopharyngeal microbiome, metabolome, and type 2 cytokines levels) | Clustering approachMetabolomic, respiratory viruses, and proteins profile | Interrelationship between rhinovirus species, major bacteria species, metabolomic profiles, and type 2 cytokines in the nasopharyngeal airway | Incident asthma by five years | Infants with endotype (atopic, rhinovirus C-positive, high nasopharyngeal levels of Moraxella catarrhalis and increased levels of type 2 cytokines) have a higher risk to develop asthma by five yearsOR: 3.74; 95% CI: 1.21–12.6; p=0.03 |
| Raita Y. et al., 202117 | Bronchiolitis cohort study | 221 infants with RSV-induced severe bronchiolitis randomly selected out of 921 infants from the MARC-35 cohort study | Nasopharyngeal airway samples (coinfection with rhinovirus, microbiome, metabolome, and transcriptome) | Clustering approachMetabolomic, transcriptomic, respiratory virus, and bacteria profile | Interrelationship between RSV infection, RSV and rhinovirus double infection, major bacteria species, metabolomic profiles, and immune mediators’ levels | Incident asthma by five years | Infants with endotype (atopic, nasopharyngeal high levels of Streptococcus pneumoniae, Moraxella catarrhalis, and increased IFN-α and -γ gene transcription) have a higher risk to develop asthma by five yearsOR: 6.00; 95% CI: 2.08–21.9; p=0.002 |
| Fujiogi M. et al., 202245 | Bronchiolitis cohort study | 917 infants with severe bronchiolitis randomly selected out of 921 infants from the MARC-35 cohort study | Nasopharyngeal airway samples (respiratory virus type, nasopharyngeal airway transcriptome, metabolome, and lipidome) | Clustering approachTranscriptomic, metabolomic, and lipidomic profiles | Interrelationship between respiratory viruses, nasopharyngeal airway transcriptomic, metabolomic, and lipidomic profiles | Incident asthma by six years | Infants with endotype (atopic, higher proportion of rhinovirus infection, lgE sensitization, low sphingolipids, and lower abundance of polyunsaturated fatty acids) have a higher risk to develop asthma by six yearsunadj OR: 3.60; 95% CI: 2.31–5.62; p<0.001 |
| Raita Y. et al., 202271 | Bronchiolitis cohort study | 244 infants with severe bronchiolitisrandomly selected out of 921 infants from the MARC-35 cohort study | Nasopharyngeal airway samples (respiratory virus type, nasopharyngeal airway transcriptome, and metatranscriptome) | Non-clustering approachTranscriptomic and metatranscriptomic profiles | Interrelationship between respiratory viruses, nasopharyngeal airway transcriptomic, and metatranscriptomic profiles | Incident asthma by six years | Infants with endotype B (RSV-rhinovirus positive, higher levels of Haemophilus influenzae, downregulated type I IFN pathways, upregulated T helper-17 pathways) have a higher risk of developing asthma by six yearsadj OR: 2.81; 95% CI: 1.11–7.26; p<0.05 |
| Zhu Z. et al., 202214 | Bronchiolitis cohort study | 244 infants with severe bronchiolitisrandomly selected out of 921 infants from the MARC-35 cohort study | Nasopharyngeal airway samples (respiratory virus type, nasopharyngeal airway transcriptome, metabolome, and metatranscriptome) | Non-clustering approachTranscriptomic, metatranscriptomic, and metabolomic profiles | Interrelationship between respiratory viruses, nasopharyngeal airway transcription, metatranscriptomic, and metabolomic profiles | Incident asthma by six years | Infants with rhinovirus-negative bronchiolitis, high levels of Streptococcus pneumoniae, lower abundance of fatty acid-related metabolites, and decreased type I and II IFN transcription are associated with a higher risk of developing asthma by six yearsFDR<0.05 for transcriptome pathways and associations between Streptococcus pneumoniae and differentially expressed transcripts |
| Zhu Z. et al., 202376 | Bronchiolitis cohort study | 575 infants with severe bronchiolitis randomly selected out of 921 infants from the MARC-35 cohort study | Nasal miRNA (respiratory virus type, differentially expressed miRNAs detected in nasal swab samples) | Non-clustering approach | Interrelationship between respiratory viruses, nasal airway miRNA, and mRNA levels | Incident asthma by six years | Differentially expressed miRNAs were negatively correlated with their mRNA targets (e.g., hsa-miR-324-3p/IL13), which were enriched in asthma-related pathwaysFDR<0.05 |
| Zhu Z. et al., 202224 | Bronchiolitis cohort study | 914 infants with severe bronchiolitis randomly selected out of 921 infants from the MARC-35 cohort study | Nasopharyngeal airway samples (respiratory virus type, nasopharyngeal airway metabolome) | Clustering approachTranscriptomic, metabolomic, and respiratory virus profile | Interrelationship between respiratory virus type and nasopharyngeal metabolomic profiles | Incident asthma by six years | Infants with endotype (lower abundance of fatty acid-related metabolites, downregulated type I and II IFN) have a higher risk of developing asthma by six yearsadj OR: 2.22; 95% CI: 1.07–4.69; p<0.05 |
| Zhu Z. et al., 202376 | Bronchiolitis cohort study | 575 infants with severe bronchiolitis randomly selected out of 921 infants from the MARC-35 cohort study | Nasal miRNA (respiratory virus type, differentially expressed miRNAs detected in nasal swab samples) | Non-clustering approach | Interrelationship between respiratory viruses, nasal airway miRNA, and mRNA levels | Incident asthma by six years | Differentially expressed miRNAs were negatively correlated with their mRNA targets (e.g., hsa-miR-324-3p/IL13), which were enriched in asthma-related pathwaysFDR<0.05 |
| Fujiogi M. et al., 202116 | Bronchiolitis cohort study | 140 infants with severe bronchiolitis randomly selected out of 921 infants from the MARC-35 cohort study | Nasopharyngeal airway and peripheral blood samples (infection with RSV or rhinovirus, nasopharyngeal and serum metabolome) | Clustering approachNasopharyngeal, serum metabolomic, and respiratory virus profiles | Interrelationship between RSV and rhinovirus infection, nasopharyngeal and serum metabolomic profiles | Incident asthma by five years | Integrated upregulated nasopharyngeal-serum carnitine metabolic pathways associated with incident asthma by five yearsadj OR: 1.48; 95% CI: 1.11–1.99; p<0.05 |
| Kyo M. et al., 202270 | Bronchiolitis cohort study | 1013 infants with severe bronchiolitis 1013 infants with nasopharyngeal metabolomic profiling data140 infants with serum metabolomic profiling data | Nasopharyngeal airway and peripheral blood samples (nasopharyngeal and serum glutathione-related metabolites) | Non-clustering approachTranscriptomic, metatranscriptomic, and respiratory virus profile | Correlations between respiratory viruses, nasopharyngeal metatranscriptome, and metabolomic profiles | Incident asthma by six years | Nasopharyngeal glutathione-related metabolic signature negatively correlated with incident asthma by six yearsadj OR: 0.90; 95%CI: 0.82–0.99; p=0.04 |
| Torgerson D.G. et al., 201577 | Bronchiolitis cohort study | 206 infants with severe bronchiolitis from the RBEL study | Peripheral blood samples (gene variants) | Non-clustering approachGenomic profiling | Correlations between respiratory viruses and gene variants | Incident asthma by seven years | Single variants in ADRB2, FLG, and NCAM1 genes in European Americans and NOS1 in African Americans were associated with an increased risk for incident asthma by seven yearsp=4.6×10−4, 1.9×10−13, 5.0×10−5, and 2.3×10−11 respectively |
| Törmänen S. et al., 201778 | Bronchiolitis cohort study | 166 infants with severe bronchiolitis | Peripheral blood samples (SNPs in Toll-like receptor genes) | Non-clustering approachGenomic profiling | Correlations between respiratory viruses and gene variants | Incident asthma by seven years | The variant TLR10 rs4129009 and TLR7 rs179008 are associated with an increased risk for incident asthma by seven yearsp<0.05 for both |
| Ooka T. et al., 202213 | Bronchiolitis cohort study | 140 infants with severe bronchiolitis randomly selected out of 921 infants from the MARC-35 cohort study | Peripheral blood and nasopharyngeal airway samples (serum proteome and respiratory virus data) | Clustering approachProteomic and respiratory viruses profile | Interrelationship between respiratory viruses and serum proteome profiles | Incident asthma by six years | The endotype of atopic infants with rhinovirus-positive bronchiolitis and NFκB-related pathways at higher risk to develop asthma by six yearsadj OR: 4.04; 95% CI: 1.49–11.0; p=0.006 |
| Shibata R. et al., 202319 | Bronchiolitis cohort study | 1016 infants with severe bronchiolitis | Peripheral blood and nasopharyngeal airway samples (total lgE and respiratory virus data) | Clustering approachRespiratory viruses and total lgE levels | Interrelationship between respiratory viruses and total lgE levels | Incident asthma by six years | The cluster of infants with high total IgE, high RSV virus load, and low RV load) had a significantly higher risk of developing asthma by six yearsadj OR: 2.93; 95% CI: 1.02–8.43; p=0.046 |
Abbreviations: ADRB2, beta-2 adrenergic receptor; CDHR3, cadherin-related family member 3; COAST, Childhood Origins of Asthma; COPSAC, Copenhagen Prospective Studies in Asthma in Childhood; CREW, Children's Respiratory and Environmental Workgroup; FLG, filaggrin; GSDMB, gasdermin B; HR, hazards ratio; IFN, interferon; IL17A, interleukin 17A; lgE, immunoglobulin E; LC–MS, liquid chromatography mass spectrometry; MARC, Multicenter Airway Research Collaboration; NCAM1, neural cell adhesion molecule 1; OR, odds ratio; SNP, single nucleotide polymorphism; RBEL, RSV bronchiolitis in early life study; TLR, toll-like receptor.
Important findings regarding risk factors for the development of childhood asthma derive from the analysis of nasopharyngeal airway biological samples from infants with severe bronchiolitis. Specifically, an analysis of data from 244 infants with severe bronchiolitis in the MARC-35 cohort study confirmed the complexity of the interactions between the nasopharyngeal airway microbiome, host transcriptome, and metabolome in regard to the development of childhood asthma.14 Briefly, the investigators reported that an increased abundance of S. pneumoniae, particularly in infants with a non-rhinovirus infection, in combination with a downregulation of type I and II interferon (IFN) transcription, and an upregulation of T cell activation, were associated with an increased risk for asthma development by age six years. Another analysis described relevant findings in regard to IFN regulation, and further highlighted that RSV-rhinovirus coinfection, in correlation to an increased abundance of Haemophilus influenzae, and downregulation of type I IFN transcription, were associated with a higher risk for asthma development.71 In regard to whether the specific bacteria (e.g., S. pneumoniae) is a pivotal factor in these associations, there are now confirmatory findings in another US cohort.40 However, the evidence around the causal role of these bacterial species (i.e., S. pneumoniae) in subsequent asthma development remains inconclusive. For example, an important unanswered question is whether these bacteria species merely acquire a pathogenic role in hosts who have already increased susceptibility to developing asthma.73
In addition to the respiratory virus, microbiome, and transcriptome findings, MARC-35 has also identified metabolomic profiles associated with a high risk for developing childhood asthma. An analysis performed for the entire cohort (i.e., 1016 infants with severe bronchiolitis), for example, links the upregulation in nasopharyngeal glutathione metabolism to a high risk of childhood asthma.70
Another interesting approach in genomic and metabolomic data from the MARC-35 cohort indicates that researchers can utilize metabolomic profiles to further define risk for asthma development in individuals with known genetic susceptibility to asthma.18 More specifically, Ooka et al. identified significant single nucleotide polymorphisms (SNPs) within loci aligned but not definitely colocalized (i.e., colocalization posterior probability≥0.5) with known asthma-susceptibility genes (e.g., ADORA1, MUC16) being associated with dysregulation of metabolic pathways and high asthma risk.12 These findings further underscore severe bronchiolitis heterogeneity investigated holistically through clustering approaches.
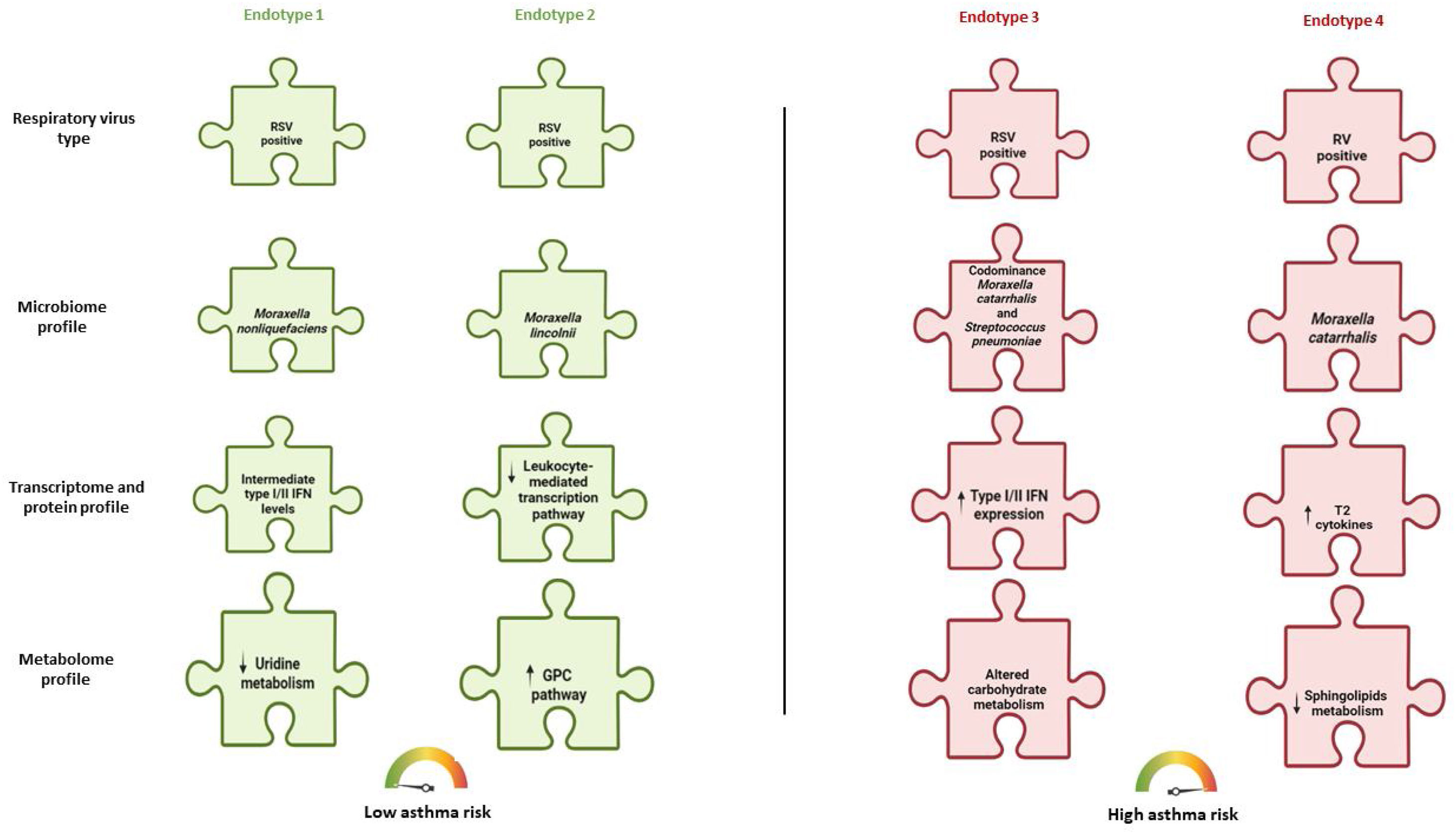
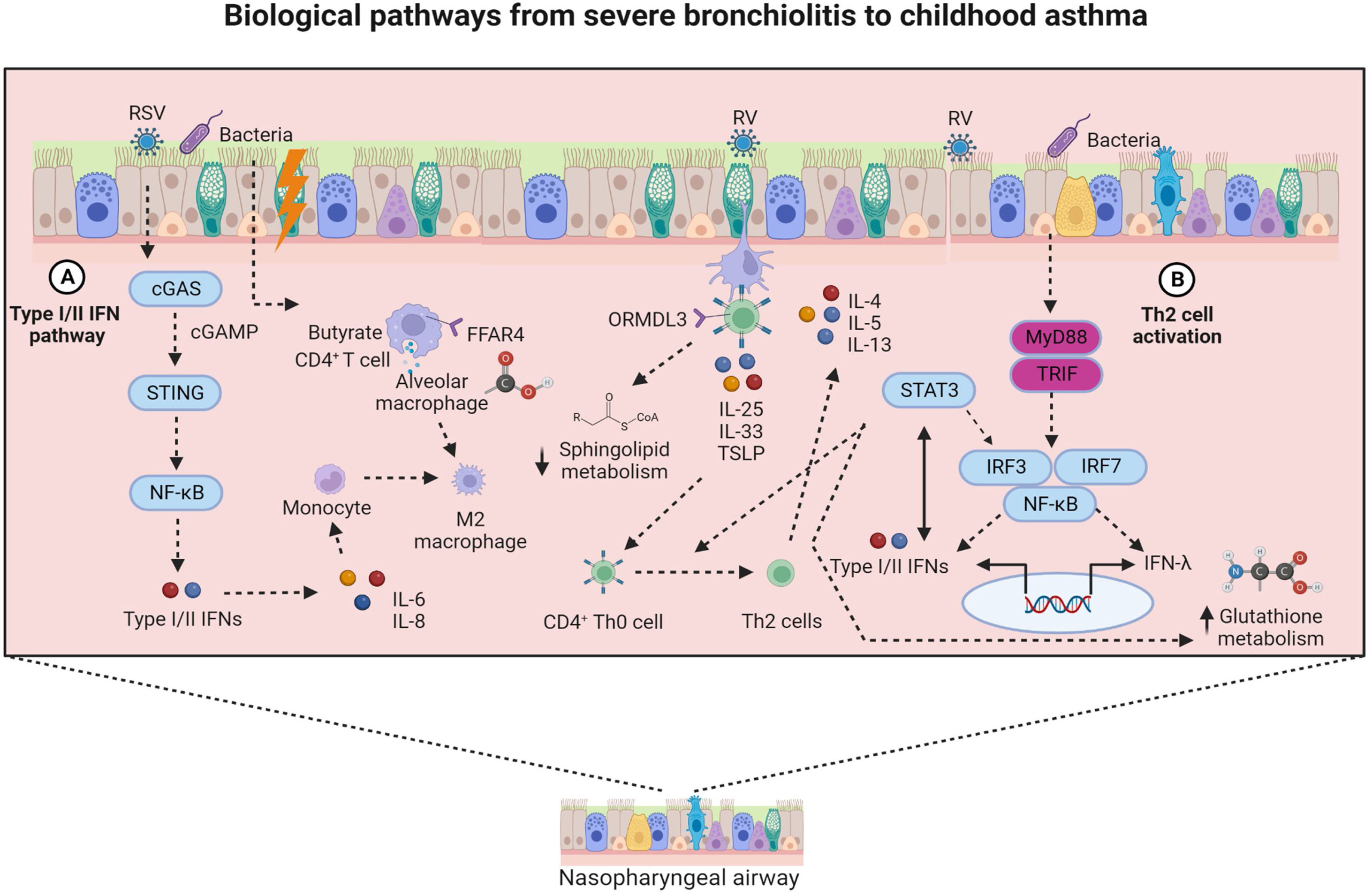
Clustering approaches (i.e., bronchiolitis endotyping)The MARC-35 cohort is the first study identifying severe bronchiolitis endotypes at high risk for asthma development. These endotypes are derived from the analyses of nasopharyngeal or peripheral blood samples or both sample types. First, there are several MARC-35 studies defining severe bronchiolitis endotypes by utilizing nasopharyngeal airway samples.17,19,23,24,45,72 The most recent of these studies applied consensus clustering approaches to clinical, virus and nasopharyngeal airway lipidomic data and identified four lipidomic endotypes at differential risks for childhood asthma.45 Out of these four endotypes, the endotype of infants with a higher proportion of parental asthma, IgE sensitization, rhinovirus infection and decreased sphingolipids metabolism was at greater asthma risk compared to other three endotypes.45 Another MARC-35 study utilizing nasopharyngeal airway samples applied a consensus clustering approach to metabolomic and transcriptomic data.24 This study identified five metabotypes at differential risks for childhood asthma. Out of these endotypes, the endotype of infants with increased nasopharyngeal amino acid metabolism and decreased fatty acid metabolism presented the highest risk for childhood asthma development. Clustering approaches were also applied in other omics data from the MARC-35 cohort.17,23 For example, the application of similarity network fusion approach to the nasopharyngeal airway microbiome, transcriptome and metabolome data, Raita et al. identified four nasopharyngeal endotypes in 221 infants with RSV bronchiolitis and another four endotypes in 122 infants with rhinovirus bronchiolitis.17,23 In RSV bronchiolitis, a high proportion of IgE sensitization and codominance of M. catarrhalis and S. Pneumoniae, in addition to increased type I/II IFN expression, were associated with the highest asthma risk.17 In rhinovirus bronchiolitis, an increased abundance of M. catarrhalis, upregulation of STAT3 transcription and type 2 cytokines were associated with the highest risk for asthma development.23 These two representative bronchiolitis endotypes at high risk for childhood asthma are depicted in Figs. 1 and 2. Fig. 2 describes in detail possible pathobiological mechanisms that underlie these severe bronchiolitis endotypes.
Severe bronchiolitis endotypes at differential risks for developing childhood asthma This figure represents a collective pattern of four severe bronchiolitis endotypes, as defined through the analysis of nasopharyngeal airway samples, at differential risks for developing childhood asthma. Each puzzle piece represents a biological profile. There are four distinct rows of puzzle pieces linking to biological profiles: respiratory viruses, microbiome, transcriptome and protein, and metabolome profile. There are four distinct columns (i.e., four sets) of puzzle pieces that represent the four distinct endotypes derived from the compilation of these four biological profiles. Among the four sets of puzzle pieces, the two sets on the left side (in green color) represent endotypes at low risk for developing asthma, whilst the two other sets on the right side (in red color) represent endotypes at high risk for developing asthma. Endotype 1 represents RSV bronchiolitis with a high abundance of Moraxella nonliquefaciens, intermediate type I/II IFN levels, and downregulated uridine metabolism. Endotype 2 represents RSV bronchiolitis with a high abundance of Moraxella lincolnii, downregulated leukocyte-mediated pathway, and upregulated GPC metabolism. Endotype 3 represents RSV bronchiolitis with a codominance of M. catarrhalis and S. pneumoniae, upregulated type I/II IFN expression, and altered carbohydrate metabolism. Lastly, endotype 4 represents RV bronchiolitis with a high abundance of M. catarrhalis, increased levels of T2 cytokines (e.g., IL-4, IL-5, and IL-13), and downregulated sphingolipids metabolism. Abbreviations: GPC, glycerophosphocholine; IFN, interferon; RSV, respiratory syncytial virus; RV, rhinovirus; T2, type 2.
Biological pathways from severe bronchiolitis to childhood asthma. This figure summarizes two biological pathways that may underlie the bronchiolitis endotypes at high risk of developing childhood asthma. From left to right, biological pathway A (i.e., type I/II IFN pathway) links to endotype 3. The type I/II IFN pathway involves the activation of cGAS-STING and the NF-κB-dependent type I/II IFNs and proinflammatory (i.e., IL-6, IL-8) mediators secretion. Additionally, the fatty acid receptor-alveolar macrophage pathway involves the polarization of alveolar macrophages toward an M2 phenotype following activation through FFAR-4. Biological pathway B (i.e., Th2 cell activation and STAT3-glutathione pathways) links to endotype 4. The Th2 cell activation pathway involves an epithelial-derived secretion of pro-Th2 mediators (i.e., IL-33, IL-25, and TSLP) and subsequent differentiation of CD4+ Th0 to Th2 cells. In the presence of ORMDL3 overexpression, the CD4+ Th0 to Th2 cell differentiation is enhanced. In synergy with the Th2 cell activation pathway, the STAT3-glutathione pathway involves the induction of type I/II IFNs and glutathione derivatives through STAT3- or NF-κB-mediated signaling. Abbreviations: cGAS, synthase for the second messenger cyclic GMP-AMP; cGAMP, cyclic guanosine monophosphate-adenosine monophosphate; FFAR, free fatty acid receptor; IFN, interferon; IL−, interleukin−; IRF3, interferon regulatory factor 3; IRF7, interferon regulatory factor 7; MyD88, myeloid differentiation primary response 88; NF-kB, nuclear factor kappa beta; ORMDL3, orosomucoid-like 3; STAT3, signal transducer and activator of transcription 3; STING, stimulator of interferon genes; Th0, T helper 0; TLR, toll-like receptor; TRIF, toll/interleukin-1R domain-containing adapter-inducing interferon-β; TSLP, thymic stromal lymphopoietin.
In addition to these MARC-35 studies that utilize nasopharyngeal airway samples, another MARC-35 study examined peripheral serum samples for endotyping.13 Ooka et al. applied clustering approaches to integrate clinical, respiratory virus and serum proteome data in 140 infants with severe bronchiolitis from MARC-35. The study identified two endotypes at highest asthma risk: (A) the endotype of rhinovirus-positive, atopic infants with dysregulated nuclear factor kappa beta signaling pathways; and (B) the endotype of RSV- and rhinovirus-double positive non-atopic infants with dysregulated TNF-α signaling pathways.13
Closing sectionAlthough the endotypes described above are associated with various degrees of risk for developing childhood asthma, some biological pathways have been shown to play a central role in the definition of more than one of these severe bronchiolitis endotypes (e.g., variability in ORMDL3 gene and associated sphingolipid metabolism, type I and II IFN regulation, fatty acid and amino acid metabolism).17,23,46,59 Lastly, we recognize that the bronchiolitis endotypes in particular may not be mutually exclusive.
It is important to remember that MARC-35 is a severe bronchiolitis cohort. Therefore, attempts to draw direct comparisons between the MARC-35 cohort and other cohorts, such as the general birth cohorts, are challenging. However, some biological pathways have been shown to play a central role in the definition of more than one of these severe bronchiolitis endotypes. These pathways include the variability in ORMDL3 gene and associated sphingolipid metabolism, type I and II IFN regulation, fatty acid and amino acid metabolism pathways.17,23,24 We also want to stress that these severe bronchiolitis endotypes derive from a single cohort and, therefore, warrant further validation, either in another cohort or in functional assays.
SummaryWith severe bronchiolitis manifesting as a heterogeneous disease, the quest to define endotypes through the analysis of omics data will help us better assimilate the complexity of this condition. Thus far, bronchiolitis cohort studies have identified distinct severe bronchiolitis endotypes that have differential risks for developing childhood asthma.13,16 At this juncture, we believe that there are two representative nasopharyngeal airway endotypes at high risk for childhood asthma development. Specifically, the first representative endotype refers to infants with severe RSV bronchiolitis, a codominance of M. catarrhalis and S. pneumoniae, upregulated type I/II IFN expression, and altered carbohydrate (i.e., increased mannitol or sorbitol) metabolism. The other endotype refers to infants with severe rhinovirus bronchiolitis, a high abundance of M. catarrhalis, upregulated type 2 cytokines, and downregulated sphingolipids metabolism.
With the current data, it remains a challenge to link these endotypes to future precision medicine applications to the management of severe bronchiolitis or to the primary prevention of asthma. However, this scoping review suggests that there are possible approaches to address this challenge. The first approach includes the validation of any identified endotypes in other birth or bronchiolitis cohort studies. The second approach includes the mechanistic validation of endotype-related findings. These approaches will lead to the identification and validation of novel targets for the treatment of severe bronchiolitis, the most common cause of infant hospitalization in the US1 and a condition still treated with non-specific interventions.74 The proposed work also may provide novel biomarkers for the development of childhood asthma, and thereby facilitate early initiation of asthma treatment for children who develop asthma. More importantly these biomarkers may guide us to novel interventions that would greatly enhance the primary prevention of childhood asthma.
Conflict of interestsThe authors state that they have no conflict of interests.