Chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD) frequently coexist, increasing the prevalence of both entities and impacting on symptoms and prognosis. CVD should be suspected in patients with COPD who have high/very high risk scores on validated scales, frequent exacerbations, precordial pain, disproportionate dyspnea, or palpitations. They should be referred to cardiology if they have palpitations of unknown cause or angina pain. COPD should be suspected in patients with CVD if they have recurrent bronchitis, cough and expectoration, or disproportionate dyspnea. They should be referred to a pulmonologist if they have rhonchi or wheezing, air trapping, emphysema, or signs of chronic bronchitis. Treatment of COPD in cardiovascular patients should include long-acting muscarinic receptor antagonists (LAMA) or long-acting beta-agonists (LABA) in low-risk or high-risk non-exacerbators, and LAMA/LABA/inhaled corticosteroids in exacerbators who are not controlled with bronchodilators. Cardioselective beta-blockers should be favored in patients with CVD, the long-term need for amiodarone should be assessed, and antiplatelet drugs should be maintained if indicated.
Chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD) frequently coexist.1–3 Patients with COPD have a 2–5-fold higher risk of ischemic heart disease, heart failure (HF), arrhythmias or peripheral vascular disease4 than individuals without COPD, while concomitancy confers a worse health status, a higher risk of hospitalization, and a worse prognosis.1,5 In patients with established CVD, COPD adjusted for cardiovascular risk factors (CVRF) is twice as prevalent as in the general population.1,2,6
The coexistence of both entities complicates the diagnostic process. Apart from common risk factors (aging, smoking, reduced lung function, sedentary lifestyle),4,7,8 patients share similar signs and findings in complementary examinations. Pathogenic mechanisms and adverse effects also overlap, confounding management.9–12
Nevertheless, no guidelines are available on CVD screening in respiratory patients, a situation that has been identified as a challenge to be met.13 The underdiagnosis of COPD or CVD when both pathologies coexist is another problem. Soriano et al.14 reported that COPD was underdiagnosed in 60% of patients with CVD and in 87% of patients with ischemic heart disease. A high prevalence of undiagnosed established CVD was also observed in another cohort of hospitalized COPD patients.15
This document, sponsored by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Spanish Society of Cardiology (SEC), reviews the prevalence and impact of CVD and COPD, highlights clinical challenges in care, and offers recommendations to optimize management.
EpidemiologyImpact of CVD on COPDRecent data16 have shown that the risk of HF is higher in patients with COPD than without COPD (OR 8.48).17 Likewise, in COPD patients, the risk of acute myocardial infarction (AMI) increases 4-fold (OR 4.42)17 and the risk of atrial fibrillation (AF) approximately 5-fold.18,19 COPD is also a risk factor for ventricular tachycardia and sudden cardiac death, regardless of CVRF.
CV events are the leading cause of hospitalization in COPD patients (up to 50% in smokers with mild/moderate COPD).1,20 Moreover, 55% of patients hospitalized for COPD have CVD.15 It is also estimated that up to a third of people with COPD die from CVD, and this figure rises to 40% in the case of previous CVD.1,21
COPD exacerbation syndrome (CES) describes a period of increased cardiocirculatory demand during which a clinical event may suddenly occur. COPD often co-exists with HF, ischemic heart disease or arrhythmias during CES, especially in patients who require hospitalization, and is associated with increased morbidity and mortality.11,12,22,23 In patients with moderate COPD and previous CVD, the risk of CV events is increased, especially within 30 days of a CES (HR 3.8), and remains elevated for 12 months. If the exacerbation requires hospitalization, the risk of a CV event in the first 30 days post-CES increases almost 10-fold (HR 9.9).12
Impact of COPD on CVDAn analysis of more than 1 million patients (29,870 with COPD) attending primary care centers revealed that the prevalence of AMI was 3.5 times higher in individuals with COPD than without COPD (HR 3.53).24 In the ALICE study that included patients with ischemic heart disease diagnosed in outpatient clinics, undiagnosed airflow obstruction was observed in one third of all participants and moderate/severe obstruction in 1 out of 5 (although one third of the obstruction patients had concomitant HF).25 This obstruction was associated with the presence of respiratory symptoms, poor health status, and visits to the emergency room.
In patients with HF, the Cardiovascular Health Study detected a prevalence of COPD of 20%, compared to 13% in the general population,6 although other authors have described rates of up to 30%.1 In a sub-study of the PARADIGM-HF trial (patients with HF and reduced left ventricular ejection fraction [LVEF] in stable phase), the presence of COPD was associated with worse health status and an increased risk of hospitalizations for CV and non-CV causes.26
In the case of AF, prevalence is generally estimated at between 10% and 20%.1
Pathophysiological mechanismsRisk factorsSmoking induces an abnormal pulmonary and systemic inflammatory response and is the most important risk factor for COPD.27 It is also involved in the genesis and instability of the atheroma plaque and the development of HF.28,29 Accelerated aging, sedentary lifestyle and abnormalities in lung development are also common risk factors.4,5 Patients who do not achieve full lung function at 25–40 years of age subsequently present a higher prevalence of respiratory, metabolic and CV diseases, as well as premature mortality.30
Pathophysiological mechanisms (Fig. S19–12)- •
Chronic systemic inflammation: COPD is characterized by an increase in macrophages, neutrophils and CD8+ T lymphocytes, which release pro-inflammatory mediators.31 Some markers, such as C-reactive protein (CRP), fibrinogen, interleukin-6, and interleukin-8, are also elevated in patients with atherosclerosis, suggesting a state of systemic inflammation that could contribute to the development of CVD.32 Endothelial dysfunction derived from neutrophil infiltration is associated with the development of atherosclerosis, which can, in turn, lead to CVD.33 Some COPD patients have elevated eosinophil levels, associated with better response to inhaled corticosteroids (ICS).31 Eosinophils also appear to be involved in atherosclerosis. They are usually absent if the plaques are stable but are detected when they rupture.34 Experimental studies have attributed a certain atheroprotective effect to interleukin-5, associated with the recruitment, maturation and proliferation of eosinophils.35
- •
Oxidative stress: Oxidative stress plays a key role in COPD-related inflammation, triggering antibody generation, nuclear damage, cellular senescence, and corticosteroid resistance.31
- •
Hypoxia: Hypoxia induces vascular remodeling and endothelial dysfunction,36 increased vascular resistance, and increases the risk of atherosclerosis.
- •
Hyperinflation: Hyperinflation is associated with reduced ventricular filling and cardiac output, and increased retrograde pressure.37–39
The CV risk is usually high in COPD patients. The European Society of Cardiology guidelines on CVD prevention40 recommend estimating the 10-year CV risk in these patients using SCORE2 tables (Table S1), classifying the following factors as high/very high risk: established CVD, diabetes mellitus, moderate/severe liver disease, and rare/genetic blood pressure disorders.
Key points for the identification, referral and management of patients with COPD and CVD.
| Patients with COPD at risk of developing CVD | |
|---|---|
| When should we suspect CVD in COPD patients? | When should we refer the COPD patient to cardiology? |
| • CVD should be suspected in patients with high/very high risk of CVD (SCORE2 if <70 years and SCORE2-OP if ≥70 years),83,84 with a history of frequent exacerbations, or who present with precordial pain, dyspnea disproportionate to lung function, or palpitations.• In the case of suspected HF, dyspnea is uninformative and an ECG, chest X-ray and blood test, including natriuretic peptide levels, will be required for differential diagnosis. In most cases, diagnostic confirmation requires an echocardiogram. Cardiac magnetic resonance imaging or cardiopulmonary stress testing may also be helpful.• In a patient with CES in whom ischemic heart disease is suspected, an ECG and clinical laboratory tests with blood markers for myocardial ischemia (cTn) must be requested. | • Patients with palpitations of unknown cause or chest pain with characteristics of angina should be referred to cardiology, especially if these symptoms are accompanied by ECG changes or elevated cTn on blood tests.• Patients with dyspnea disproportionate to their lung function who present high central venous pressure, edema and crackling, any significant ECG change, findings on chest X-ray or elevated natriuretic peptides should also be referred to cardiology. |
| Patients with CVD at risk of developing COPD | |
|---|---|
| When should we suspect COPD in patients with stable CVD? | When should we refer the CVD patient to respiratory medicine? |
| • COPD should be suspected in any stable CVD patient who has recurrent bronchitis, cough and expectoration or dyspnea that is not attributable to heart disease.• Lung function evaluation should include forced spirometry (except in decompensations of underlying heart disease), imaging tests, and complete clinical laboratory tests including C-reactive protein (useful in CES).In patients with stable HF, carbon monoxide diffusing capacity should also be determined. | • Patients with stable CVD, rhonchi or wheezing on auscultation (especially if they have a history of smoking or other risk factors for COPD), patients with evidence of air trapping or chronic bronchitis on chest X-ray, and patients with pulmonary emphysema on coronary computed tomography should be referred to respiratory medicine |
| Management of COPD and CVD patients | |
|---|---|
| Pharmacological treatment of stable COPD in the CVD patient | Pharmacological treatment of CVD in the COPD patient |
| • Low-risk or high-risk non-exacerbators49: long-acting bronchodilators (LAMA or LABA), alone or in combination, to improve lung function, control symptoms, and prevent exacerbations.85,86• In acute patients not controlled with bronchodilator therapy: triple therapy (ICS/LAMA/LABA) to reduce the number of exacerbations, prevent hospitalizations, and reduce all-cause mortality57,58,74 and CV mortality.57,58 | • Patients with CVD or high risk of CV complications: preferably cardioselective beta-blockers.66,67• Evaluate the need for long-term amiodarone with the pulmonologist.73• Maintain the use of antiplatelets in patients with that indication.81,82 |
CES: COPD exacerbation syndrome; COPD: chronic obstructive pulmonary disease; cTn: cardiac troponin; CV: cardiovascular; CVD: cardiovascular disease; ECG: electrocardiogram; HF: heart failure; ICS: inhaled corticosteroids; LABA: long-acting β2-adrenergic agents; LAMA: long-acting antimuscarinic agents.
Clinical situations that determine which COPD patients need a more rigorous CV study include: precordial pain, dyspnea disproportionate to lung function, frequent exacerbations, lipid profile changes, altered inflammatory parameters (CRP) or biomarkers (ultrasensitive troponin T and N-terminal fraction of pro-B-type naturietic propeptide [NT-proBNP]), severe obstruction, air trapping, pulmonary hyperinflation, and reduced LVEF.
Tests for the study of CVD in patients with stable COPD (Table S2)In patients with previous CVD, high-sensitivity cardiac troponin (hs-cTn) helps determine CV risk.41 Levels>7.7ng/l in patients with stable COPD are associated with a higher risk of future CVD,41 while even higher levels are seen in older patients with more CV comorbidities and evidence of metabolic syndrome. When there is no established history of CVD, biomarkers are not a reliable indication of subclinical CVD. A study that analyzed hs-cTn concentrations>5ng/l associated with signs of myocardial ischemia on electrocardiogram found a 4-fold risk of death in COPD patients, regardless of their severity. A similar increase in risk was observed among COPD patients without known ischemic heart disease. However, the usefulness of these biomarkers should be further assessed.42
The characteristic symptoms of HF (orthopnea, paroxysmal nocturnal dyspnea, hepatojugular reflux, and lower limb edema) should be evaluated in COPD patients. Dyspnea is the main symptom in HF and COPD and should be assessed as part of a differential diagnosis43 that should consist of electrocardiogram, chest X-ray, and blood tests, including natriuretic peptide (NP). The accuracy of NP for the diagnosis of HF was evaluated in a study with 200 patients with stable COPD.44 The “optimal” cut-off points for excluding HF with a negative predictive value (NPV) of 0.95 were 125pg/ml for NT-proBNP and 35pg/ml for B-type natriuretic peptide (BNP). However, in COPD, NPs show less diagnostic accuracy than in patients with dyspnea without COPD.45 Elevated systolic pressure and right ventricular dysfunction associated with COPD may increase NT-proBNP levels in the absence of left ventricular (LV) dysfunction. NT-proBNP is also elevated in the presence of renal failure, AF, and in older patients, situations that are common in COPD. NT-proBNP may also have a prognostic value in stable COPD. Indeed, levels>500pg/ml predicted 1-year mortality in patients with preserved LVEF undergoing major vascular surgery (HR 7.7). A recent meta-analysis has shown that elevated NT-proBNP values are associated with an increased risk of all-cause mortality in COPD patients with and without exacerbations (HR 2.87 and 3.34 respectively).46
If HF is suspected on electrocardiogram, chest X-ray or clinical laboratory testing, an echocardiogram should be requested to confirm/exclude the diagnosis, although some patients may have a poor echocardiographic window.
Cardiac magnetic resonance imaging may also be useful in patients with COPD and suspected HF. Air trapping and hyperinflation are associated with a higher LV mass39 and are linked with a higher risk of HF and CV death.
When symptoms associated with exertion are disproportionate to lung function, cardiopulmonary exercise testing (CPET) can detect coexisting diseases (myocardial ischemia, pulmonary vascular disease, or musculoskeletal dysfunction).47
Identifying patients with CES in whom CVD may be suspectedRecognizing AMI is a complex task in patients with CES, since they very frequently present atypical chest pain or dyspnea. COPD patients are also more likely to present unstable angina or non-ST elevation AMI than individuals without COPD, increasing the risk of misdiagnosis and treatment delay.
AF is an independent predictor of hospital mortality that is directly related to FEV1 and often precipitated by a CES.
Tests for the study of CVD in patients with CESWhen ischemic heart disease is suspected in patients with CES, an electrocardiogram should be performed and blood markers of myocardial ischemia (troponins and kinetics) determined.14 cTn is elevated in 18–27% of CES requiring hospitalization and is an independent predictor of exacerbation severity and long-term mortality.
In patients with CES, NPs have a high NPV for ruling out HF in highly symptomatic patients that present with dyspnea in the emergency room. BNP values<100pg/ml or NT-proBNP<300pg/ml exclude HF with a NPV of >90%.3
Referral from respiratory medicine to cardiology (Table S3)Criteria for referring stable COPD patients in whom CVD is suspectedAccording to patient symptoms:
- a)
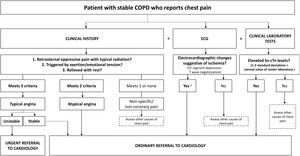
Chest pain: Patients with angina chest pain should be referred, particularly if changes are also observed in the electrocardiogram or if the patient has had raised troponins in a previous test (Fig. 1a).
Fig. 1.Algorithms for referral from respiratory medicine to cardiology of patients with stable COPD and (a) chest pain (suspected concomitant ischemic heart disease), (b) dyspnea disproportionate to their lung function (suspected concomitant HF), or (c) palpitations (suspected arrhythmia). a In COPD patients with signs of myocardial ischemia on ECG, hs-cTn concentrations>5ng/l have been associated with a 4-fold risk of death; b Take in account any ECG changes; a normal ECG generally rules out a diagnosis of HF; c Hawkins NM, et al. BMC Pulm Med 2017;17(1):1144; d Atrial fibrillation, atrial flutter, atrial tachycardia, intranodal tachycardia; e Especially if they are frequent, coupled with a normal sinus beat (bigeminy trigeminy), doublets, triplets or originate in multiple foci (of different morphology in 1 lead); f Wolff–Parkinson–White Syndrome. AV: atrioventricular; BNP: B-type natriuretic peptide; chest Rx: chest X-ray; COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease; ECG: electrocardiogram; HF: heart failure; hs-cTn: high-sensitivity cardiac troponin; IHD: ischemic heart disease; NP: natriuretic peptides; NPV: negative predictive value; NT-proBNP: N-terminal fraction of pro-B-type naturietic propeptide; TSH: thyroid-stimulating hormone.
Given the association between CES and the onset, days or weeks later, of ischemic heart disease, attention should be paid to suspicious symptoms or electrocardiographic changes suggestive of ischemia after a CES. Evaluation by a cardiologist and an ischemia stress test are recommended. If the acoustic window allows, a dobutamine stress echocardiogram is preferable. In patients with significant COPD, the conventional stress test is usually inconclusive and a resting MIBI scan with vasodilators may be contraindicated due to the risk of bronchoconstriction.
- b)
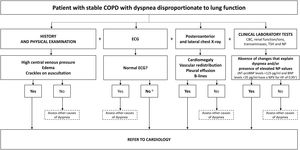
Dyspnea: Most patients with COPD have dyspnea, and a differential diagnosis should be made to rule out other causes (Table 2). Fig. 1b shows a cardiology referral algorithm for COPD patients with dyspnea disproportionate to lung function (suspected HF).48
Table 2.Differential diagnosis of dyspnea: respiratory or cardiac origin.
Respiratory Cardiac (HF) Medical history Long and recurring Progressive Lung auscultation Rhonchi and wheezing Crackles Cardiac auscultation Muffled heart sounds Gallop, murmurs Chest RX, cardiac silhouette Normal Cardiomegaly Chest X, pulmonary parenchyma Air trappingInterstitial patternResidual lesions Interstitial edema, alveolar edema Chest X-ray, vessels Pulmonary hypertension Vascular redistributionVeno-capillary hypertension ECG Right cavity overload, low voltage, RBBB LVH, ischemia-necrosis, LBBB Spirometry Obstruction Normal or mild restriction Clinical improvement with diuretics No Yes Response to bronchodilators Yes No ECG: electrocardiogram; LBBB: complete left bundle branch block; LVI: left ventricular hypertrophy; RBBB: complete right bundle branch block; Rx: X-ray.
Adapted from Table 5 in Murga N et al., 2015.87 - c)
Palpitations: Fig. 1c shows a referral algorithm for COPD patients who report palpitations.
- d)
Syncope: Key elements for the diagnosis of syncope are medical history, examination (including measurement of decubitus/standing BP), and a 12-lead electrocardiogram. Depending on the results, recommendations are as follows:
- •
Do not refer patients with occasional neurally mediated syncope and normal electrocardiogram.
- •
Make normal priority referral in patients with very frequent/decapacitating neurally mediated syncope or if the etiology is unclear (does not meet all criteria for benignity).
- •
Make high priority referral in patients with suspected heart disease who have had non-recent syncope (more than 72h) or show changes in baseline electrocardiogram.
- •
Make urgent referral to the emergency room in patients with heart disease and recent syncope (less than 72h), arrhythmia as a cause of syncope, or hemodynamic instability.
According to physical examination findings:
Heart murmurs: Patients with diastolic and systolic murmurs≥grade III, other symptoms/signs of valvular disease, or findings on chest X-ray should be referred to cardiology for echocardiography.
According to findings in complementary tests:
- a)
Coronary calcifications on computed tomography (CT): Severity is associated with an increased risk of CV events. In case of symptoms consistent with ischemic heart disease, the patient should be referred to cardiology and managed as probable concomitant heart disease. In the absence of symptoms, incremental CV risks should be evaluated and, in case of high/very high risk, the patient should be referred to cardiology for further study.
- b)
Transthoracic echocardiogram findings not related to lung disease (right ventricular dilation, tricuspid valve insufficiency, estimated degree of ventricular hypertrophy, etc.).
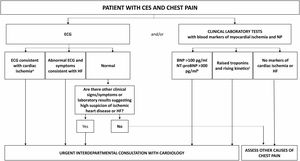
Fig. 2 shows an algorithm for referring patients with CES and chest pain to cardiology.
Algorithm for referral from respiratory medicine to cardiology of a patient with CES and chest pain. a Take into account that patients with COPD are more likely to present unstable angina or non-ST-elevation AMI than patients without COPD; b values of BNP<100pg/ml or NT-proBNP<300pg/ml rule out a diagnosis of HF with a negative predictive value greater than 90%; c cTn is elevated in 18–27% of CES requiring hospitalization. High values do not necessarily imply acute myocardial ischemia requiring specific intervention or treatment. AMI: acute myocardial infarction; BNP: B-type natriuretic peptide; ECG: electrocardiogram; CES: COPD exacerbation syndrome; COPD: chronic obstructive pulmonary disease; cTn: cardiac troponin; HF: heart failure; NP: natriuretic peptides; NT-proBNP: N-terminal fraction of pro-B-type naturietic propeptide.
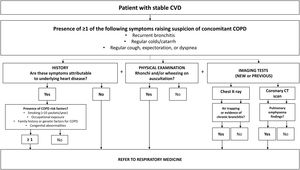
The diagnostic suspicion begins in an adult with CV with a history of smoking or chronic exposure to inhaled toxins who presents with respiratory symptoms.49
Tests for the study of COPD in patients with stable CVDIn patients with ischemic heart disease, the presence of recurrent bronchitis, cough, expectoration or dyspnea not attributable to heart disease requires pulmonary assessment, including forced spirometry and chest CT in cases of severe dyspnea that persists despite dual bronchodilation.49,50
In the ALICE study, spirometry in patients with ischemic heart disease revealed a high prevalence of undiagnosed airflow obstruction.25 Framingham risk scores were similar in participants with and without airflow obstruction, suggesting that conventional scores do not always reflect all CV risks in COPD patients. Individuals with airflow obstruction had higher levels of certain CV risk biomarkers, such as CRP.
In HF, a differential diagnosis should be made to rule out COPD in patients with dyspnea. Respiratory function tests usually show a restrictive pattern with reduced alveolar volume and carbon monoxide diffusing capacity. The 6-min walk test is also useful for assessing stress tolerance and determining if hypoxemia on exertion contributes to decreased functional capacity. When dyspnea is not explained by lung or heart studies, it is reasonable to perform a cardiopulmonary exercise test.47
Identifying patients with decompensated CVD who may have COPDDiagnosing COPD in patients admitted for HF can be a challenge, especially in individuals with elevated NP, AF, pulmonary artery hypertension, and RV overload. Fluid retention in HF may worsen airflow obstruction and hypoxemia, and the tachycardia associated with CES may precipitate HF decompensation.51
Tests for the study of COPD in patients with decompensated CVDSpirometry testing during exacerbations in patients with acute coronary syndrome or HF is inappropriate, since edema of the lung or bronchial wall causes ventilatory pattern changes that in many cases only normalize weeks after diuretic treatment.
Chest X-ray or coronary CT scans can help detect pulmonary hyperinflation or emphysema that would suggest a diagnosis of COPD which should be confirmed, when possible, by spirometry. Chest CT may be indicated for a more comprehensive pulmonary assessment in patients with persistent dyspnea or hypoxemia not explained by CVD. If oxygen saturation is low, arterial blood gas tests may be performed to assess the presence of hypoxemia or hypercapnia.
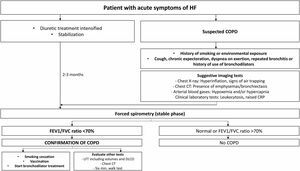
Referral from cardiology to respiratory medicineFigs. 3 and 4 show algorithms for referring patients with stable CVD and patients with decompensated CVD with suspected COPD to respiratory medicine, respectively.
Intervention algorithm for a patient with decompensated CVD and suspected COPD. CAT: computerized axial tomography; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; CVD: cardiovascular disease; DLCO: diffusing capacity for carbon monoxide; FEV1/FVC: forced expiratory volume in 1 second/forced vital capacity ratio; HF: heart failure; LFT: lung function tests.
Table S4 gives recommendations for the referral of patients with stable CVD and already diagnosed COPD to respiratory medicine in case of CES or worsening COPD.
Management of patients with COPD and CVD (Table 1)Table S5 shows the key recommendations for treating CVRF in COPD and CVD patients.
Pharmacological treatment of COPDInfluence of COPD treatment on CVDDecrease in air trapping and effect on preloadLong-acting bronchodilators are the drug of choice in COPD.49 Both LABA and LAMA are safe,52,53 but most studies exclude patients with known heart disease, especially tachyarrhythmias, so caution is always needed.54,55 These drugs reduce pulmonary hyperinflation, which could favor ventricular filling and improve cardiac output.37–39 Hohlfeld et al.56 showed that the LABA/LAMA combination reduced pulmonary residual volume and increased LV end diastolic volume, indicating an improvement in hyperinflation. However, there are no data explaining how this might affect long-term outcomes.
Reducing CV mortality by reducing the number and severity of exacerbationsTwo randomized clinical trials studying the prevention of exacerbations with triple therapy (TT; ICS/LAMA/LABA) demonstrated a lower mortality than with LAMA/LABA in COPD patients with frequent exacerbations (ETHOS and IMPACT studies).57,58 With regard to CV mortality, the ETHOS study showed 60% fewer deaths in the TT group than in the LAMA/LABA group (11 vs 28 deaths, respectively).58 The IMPACT study reported 4.2 deaths versus 8.7 deaths/1000 participants, a reduction of 52%.57 This decrease is partly explained by the reduction in the number and severity of exacerbations, inflammatory events that are associated with endothelial damage.
Decreased arterial stiffnessCOPD patients have increased arterial wall stiffness, which may be associated with increased CV risk. However, to date no pharmacological intervention has been shown to decrease this condition.36
Risk of cardiac arrhythmiasThere is strong evidence indicating that the risk of long-acting bronchodilators for CV events in general and arrhythmias in particular is similar to that of placebo.52 However, population studies have suggested an increased risk of CV events after starting pharmacological treatment, especially in elderly populations.59,60
Decompensated HFThe use of high doses of short-acting beta-agonists during COPD exacerbations, especially when administered by nebulizers, is associated with elevated biomarkers and stress cardiomyopathy, and should be avoided.61 In addition, since sympathetic stimulation and increased heart rate can lead to HF decompensation, the use of steroids and other bronchodilators should be preferred in these patients.
Acute ischemic events, infarction, and strokeIn patients with COPD and frequent exacerbations, a reduction in CV deaths has been observed among patients treated with TT vs dual bronchodilation.62,63 The mechanisms have not been clarified.
Recommendations for the treatment of COPD in patients with CVDTable 3 summarizes the main recommendations for the use of COPD treatments from the point of view of the pulmonologist and the cardiologist.
Recommendations for the use of treatments for COPD and CVD.
| Treatments for COPD | |
|---|---|
| From the point of view of the pulmonologist | |
| What to do | |
| • Prescribe treatment with a single inhaler | Since COPD and CVD are both chronic diseases, pharmacological treatment should be as simple as possible to improve the outcomes. The recommendation, as indicated by GOLD 2023, is for treatment with a single inhaler: this is more convenient and effective than multiple inhalers.74 |
| • Assess adherence, side effects and the correct inhalation technique at each follow-up visit | With the aim of obtaining greater clinical stability |
| • Prescribe long-acting bronchodilators in low-risk patients and high-risk non-exacerbators | Long-acting bronchodilators (LAMA or LABA), alone or in combination, are the treatment of choice for the low-risk or non-exacerbating patient, as they improve lung function, provide better symptom control, and decrease exacerbations more than short-acting bronchodilators.85,86 |
| • Prescribe triple therapy in exacerbators | In patients with frequent exacerbations, the use of triple therapy in a single device has been shown to reduce the number of exacerbations, prevent hospital admissions and decrease mortality from all causes.57,58,74 |
| What not to do | |
| • Prescribe treatment with short-acting bronchodilators | All patients with COPD should be treated with long-acting bronchodilators. Single-agent therapy with short-acting bronchodilators should be avoided, except for use as rescue medication, because they offer no improvement over long-acting preparations and are less cost-effective. |
| • Prescribe treatment with ICS alone or with LABA/ICS | The use of ICS in monotherapy or in combination with LABA should be avoided in COPD patients, since it may be associated with increases in mortality in the first case and because triple therapy delivered in a single device is preferable to LABA/ICS in the prevention of exacerbations. The effectiveness of ICS in triple therapy is dependent on the level of eosinophils in peripheral blood: a clinical effect is seen at >100cells/mm.3,88 |
| From the point of view of the cardiologist | |
|---|---|
| What to do | |
| • The use of fixed-dose combinations of bronchodilators or triple therapy in a single device is preferable | The use of fixed-dose combinations of bronchodilators improves adherence and clinical efficacy. The use of triple therapy could also decrease the future risk of MACE. Based on these results, triple therapy should be considered in patients with CV comorbidity, especially if they present exacerbations or their eosinophil count is >300cells/mm.3,88 |
| • Limit the use of short-acting beta-agonists for exacerbations and use the lowest effective dose | Particularly in patients with tachyarrhythmias and/or ischemic heart disease due to increased heart rate and sympathetic tone. |
| What not to do | |
| • In general, bronchodilator treatment should not be modified in patients with stable COPD | Alternatively, respiratory medicine and cardiology should establish joint protocols to ensure continuity of care in decision-making. |
| Treatments for CVD | |
|---|---|
| Impact of CVD treatments on COPD: general recommendations | |
| • Lung function | The use of both short and long term cardioselective beta-blockers is safe in COPD patients, regardless of their severity, and should not be restricted. |
| • Pulmonary toxicity | The decision to start amiodarone and its long-term use in COPD patients should be individualized and reevaluated, weighing up the risk of potential pulmonary worsening against the benefit of preventing supraventricular or ventricular arrhythmias. |
| • COPD exacerbations and mortality | The use of beta-blockers should be individualized, but given their potential benefits in COPD patients, the use of selective β1-blockers should be prioritized in patients with a previously established indication for CVD or an increased risk of CV events. |
| • Antiplatelets | Antiplatelet use should be maintained in COPD patients with a CV indication, except in the presence of hemorrhagic complications. |
| From the point of view of the cardiologist |
|---|
| What to do |
| • The use of cardioselective beta-blockers in patients with CVD or a high risk of CV complications is preferable. |
| • Evaluate the need for long-term amiodarone in COPD patients in conjunction with the pulmonologist. |
| • Maintain the use of antiplatelets in patients with an indication for CVD. |
| What not to do |
| • Prescribe non-cardioselective beta-blockers. |
| • Replace beta-blockers with other negative chronotropic drugs such as diltiazem, verapamil, or digoxin. |
| From the point of view of the pulmonologist |
|---|
| What to do |
| • Prescribe beta-blockers in patients who require them for the treatment of CVD, as their use is safe (except in the case of propranolol, which worsens FEV1) and is associated with the same beneficial effects as in the general population.74 |
| What not to do |
| • Prescribe statins to prevent exacerbations (the scientific evidence is insufficient, and quality clinical trials have shown discordant outcomes).89,90 |
| • Prescribe beta-blockers to prevent exacerbations (lack of evidence).91 |
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease; CV: cardiovascular; FEV1: forced expiratory volume in 1 second; LAMA: long-acting antimuscarinic agents; ICS: inhaled corticosteroids; LABA: long-acting β2-adrenergic agents; MACE: major adverse CV events.
The fear of exacerbating bronchospasm is one of the main reasons for avoiding beta-blockers in COPD patients, despite their benefits in individuals with HF, tachyarrhythmias, and ischemic heart disease. Theoretically, antagonism of β-2 receptors involves constriction of the smooth muscle of the airway, demonstrated in patients with asthma. However, in an analysis of 2712 patients who underwent regular spirometries over a period of 4 years, the prolonged use of beta-blockers (88% cardioselective) had no deleterious effect on FEV1 or forced vital capacity, even in patients with more severe lung disease or treated with inhaled TT. Indeed, the latter had greater reductions in mortality and exacerbations.64 In a meta-analysis of randomized studies of cardioselective beta-blockers, no effect was observed on FEV1 compared to placebo in either single or chronic doses, and the response to inhaled beta2-agonists was not affected.65 There do, however, appear to be differences between beta-blockers: cardioselective agents, particularly bisoprolol (selectivity β1/2 with a ratio of 14:1), were seen to be superior to non-selective agents.66,67 In patients with moderate or severe COPD, randomized studies with bisoprolol vs. placebo found worsening of FEV1, although symptoms and exercise capacity were not affected, and response to salbutamol was unchanged.68,69 Cardioselective beta-blockers in COPD patients, therefore, do not produce adverse respiratory effects, as concluded by a Cochrane review.70
Pulmonary toxicityOne of the most serious adverse effects of amiodarone is pulmonary toxicity, which occurs with a long-term incidence of 1–5%.71,72 It develops in a dose- and time-dependent manner and can manifest in different ways, the most frequent and severe events being interstitial pneumonitis and residual fibrosis. Risk factors include a history of lung disease and older age.73 Its use for the prevention of arrhythmias in patients who already have COPD should therefore always be reevaluated.
COPD exacerbations and mortalityBeta-blockers may be associated with a lower risk of decompensation and mortality in COPD patients, perhaps because of their reduced effect on the increase in adrenergic tone and the proinflammatory state. Although some data are discordant, individual studies and meta-analyses suggest they have an overall benefit in COPD.74 Two meta-analyses have shown a reduction in mortality of 31–36% in patients with or without known CVD.75,76 The benefit observed in these studies may be explained by the high prevalence of subclinical or underdiagnosed disease in COPD. However, a lower risk of COPD exacerbations has also been identified: in a prospective study of 3464 patients, the risk was reduced by 27%, and by 67% in patients with COPD stage GOLD 3–4 receiving home oxygen therapy.77
Heart failureTreatment of HF with reduced ejection fraction is associated with a reduction in the risk of hospitalization and CV mortality.78 During HF decompensations, if the patient shows signs of stability, background treatment should be maintained, while increasing the use of loop diuretics and continuing to monitor congestion.
In HF patients with preserved ejection fraction, symptomatic treatment should be maintained. Sodium-glucose co-transporter type 2 inhibitors are a recent addition to the therapeutic arsenal.79,80
AntiplateletsThe use of antiplatelets (particularly acetylsalicylic acid) is associated with lower mortality from any cause and better progress during admissions for COPD exacerbations, with shorter stays and less need for mechanical ventilation.81,82 It is also associated with a 19% decrease in the risk of death. Similar effects have been observed among outpatients and patients admitted for exacerbation. Current indications for antiplatelet drugs in COPD patients are similar to those in the general population.
Recommendations for the treatment of CVD in COPD patientsTable 3 lists recommendations for the use of CVD treatments, both generally and from the point of view of the cardiologist and the pulmonologist.
Future needs in cardiopulmonary riskSeveral common CVRFs also increase the chance of developing or dying from lung disease. The combination of CV and pulmonary risks could therefore constitute a “cardiopulmonary risk”, the calculation of which could help in clinical decision-making.
Moreover, the management of cardiorespiratory diseases (prevention, early diagnosis, acute illness, rehabilitation, and chronic patients) requires close collaboration among all professionals involved to increase the efficiency of the health system and avoid duplicated procedures, inconsistent approaches, and unnecessary referrals.
Authors’ contributionsThe authors have been selected by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Spanish Society of Cardiology (SEC) for their experience in the treatment of both entities, as reflected in multiple publications over the last decade. The cardiologists participate in SEC working groups, such as the heart failure association and the cardiorenal syndrome and heart failure congestion management strategies working group. The pulmonologists are active members of the COPD division and/or the SEPAR integrated COPD research program, and some of them have participated in the development of COPD guidelines (GesEPOC) and COPD recommendations (GOLD).
FundingThis work has received funding from AstraZeneca Spain that included logistical support for the meetings needed to develop this document (including booking venues, authors’ travel expenses, audiovisual equipment, etc.), and the editorial services of VMLY&R Health Spain (including compilation of the texts sent by the authors and editorial correction).
Conflict of interestsThis document was funded by AstraZeneca, which has conflicts of interest with its content. AstraZeneca has not influenced the contents of this document, which are the responsibility of the authors. The authors have not received any fees directly from AstraZeneca for the preparation of this document.
J. de Miguel has received grants and honoraria from AstraZeneca, BIAL, Boehringer, Chiesi, FAES, Gebro, GSK, Janssen, Menarini, Novartis, Roche, Teva, Pfizer and Zambón.
J. Núñez has received personal honoraria and consultancy fees from Aleviant, AstraZeneca, Boehringer Ingelheim, Bayer, Novartis, NovoNordisk, and Rovi.
S. Santos has received honoraria for lectures and advisory board participation from GSK, Chiesi, Laboratorios Menarini, and Astra Zeneca, and for lectures from MSD.
N. Manito has participated in advisory boards and given lectures for Astra Zeneca and Novartis.
B. Alcázar-Navarrete has received grants and honoraria from GSK, honoraria and non-economic support from Boehringer Ingelheim, honoraria and non-economic support from Chiesi, non-economic support from Laboratorios Menarini, grants, honoraria, and non-economic support from AstraZeneca, honoraria from Gilead, honoraria and non-economic support from MSD, honoraria from Laboratorios BIAL, and honoraria from Zambon.
J.F. Delgado has received consultancy fees from Novartis, AstraZeneca and Rovi, has conducted research projects for Novartis, Astra Zeneca, Amgen, Impulse Dynamics, Orion Pharma, and Boehringer Ingelgeim-Lilly, and has received honoraria for lectures from Novartis, Orion, Astra Zeneca, Medtronic, Rovi, Impulse Dynamics, and Boehringer Ingelheim-Lilly.
D. Pascual has received honoraria for lectures and consultancy from AstraZeneca, Novartis, Rovi, Vifor, Pfizer, and Roche Diagnostics.
P. Sobradillo has received honoraria for lectures from GSK, Chiesi, Menarini Laboratories, Astra Zeneca, BIAL Laboratories, and CSL Bering.
The authors thank AstraZeneca Spain for their logistical and financial support during the preparation of this document, and the editorial services provided by Anna Nualart and Joan Mañé of VMLY&R Health.