Identification of patients with acute symptomatic pulmonary embolism (PE) who are at low-risk for short-term complications to warrant outpatient care lacks clarity.
MethodIn order to identify patients at low-risk for 30-day all-cause and PE-related mortality, we used a cohort of haemodynamically stable patients from the RIETE registry to compare the false-negative rate of four strategies: the simplified Pulmonary Embolism Severity Index (sPESI); a modified (i.e., heart rate cutoff of 100beats/min) sPESI; and a combination of the original and the modified sPESI with computed tomography (CT)-assessed right ventricle (RV)/left ventricle (LV) ratio.
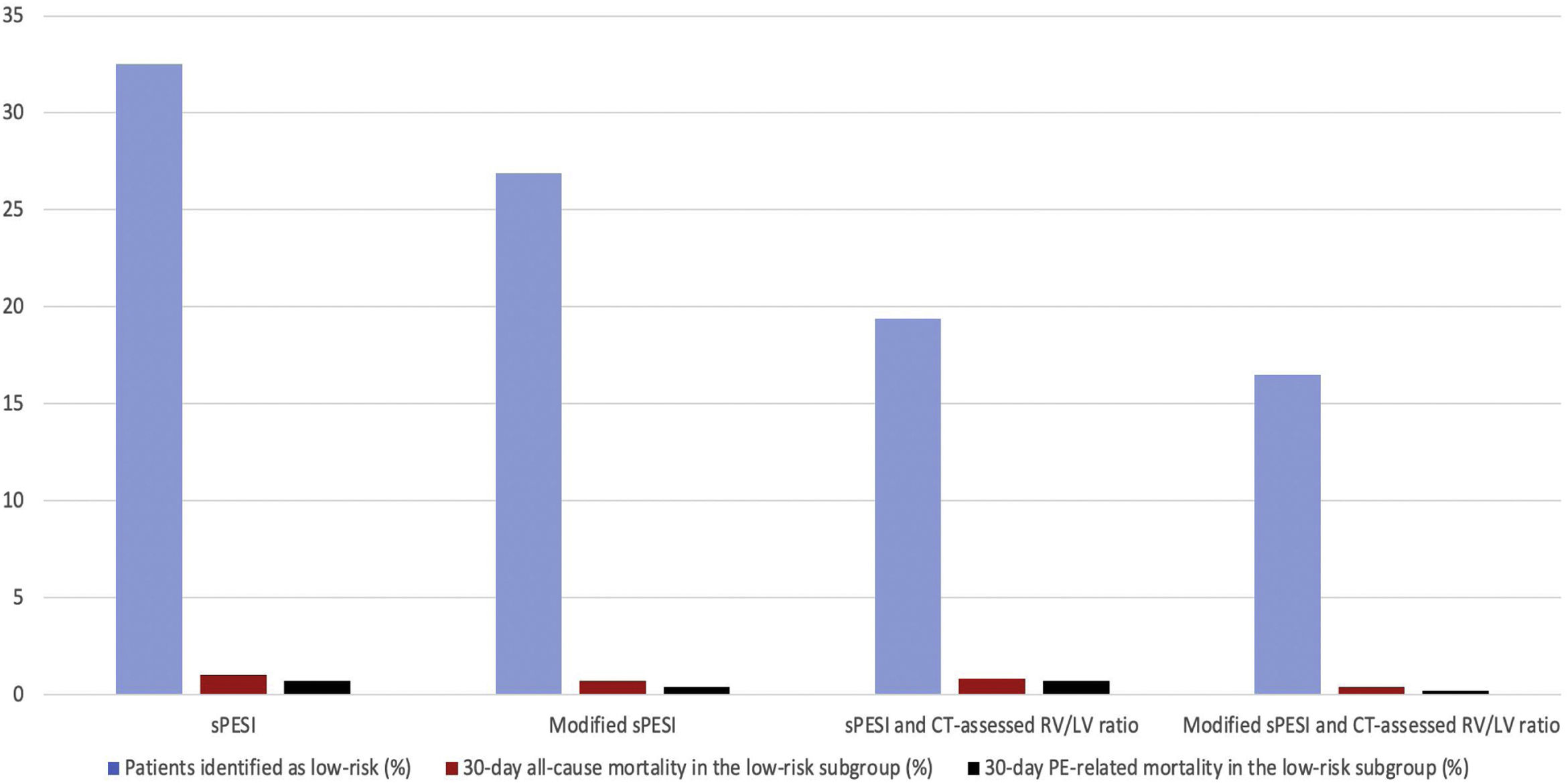
ResultsOverall, 137 of 3117 patients with acute PE (4.4%) died during the first month. Of these, 41 (1.3%) died from PE, and 96 (3.1%) died from other causes. The proportion of patients categorized as having low-risk was highest with the sPESI and lowest with the combination of a modified sPESI and CT-assessed RV/LV ratio (32.5% versus 16.5%; P<0.001). However, among patients identified as low-risk, the 30-day mortality rate was lowest with the combination of a modified sPESI and CT-assessed RV/LV ratio and highest with the sPESI (0.4% versus 1.0%; P=0.03). The 30-day PE-related mortality rates for patients designated as low-risk by the sPESI, the modified sPESI, and the combination of the original and modified sPESI with CT-assessed RV/LV ratio were 0.7%, 0.4%, 0.7%, and 0.2%, respectively.
ConclusionsThe combination of a negative modified sPESI with CT-assessed RV/LV ratio ≤1 identifies patients with acute PE who are at very low-risk for short-term mortality.
Acute pulmonary embolism (PE) represents a spectrum of clinical syndromes with varying clinical outcomes.1,2 Based on risk profiles, some patients with PE may require hospitalization, while others may safely undergo treatment at home and avoid admission to a hospital.3,4 Clinical prognostic models were developed to identify low-risk patients with PE who may be candidates for outpatient care or an abbreviated hospital stay.5,6 Particularly, the simplified Pulmonary Embolism Severity Index (sPESI) accurately identifies patients who have low-risk for all-cause mortality that occur within 30 days of PE diagnosis.6–8
However, some clinicians are still hesitant to recommend outpatient therapy of patients identified as low-risk by a clinical prognostic score. For this reason, researchers have aimed at improving the ability of the sPESI for identification of low-risk patients by modifying the cutoff value of some items in the score (e.g., heart rate), or by adding some additional prognostic tools (e.g., computed tomography [CT]-assessed right ventricular [RV] enlargement). For instance, a recent study found that the modification of the heart rate cutoff value could increase the sensitivity of the sPESI for detection of short-term mortality.9 In addition, a systematic review and meta-analysis of studies with low-risk PE patients showed that the presence of CT-assessed RV enlargement or echocardiographic RV dysfunction was associated with 4.2-fold increased risk of all-cause mortality.10 Based on the latter study, the recent European Society of Cardiology (ESC) guidelines suggest assessment of the RV by imaging methods in patients with a negative sPESI to identify low-risk PE.11
However, the usefulness of CT assessment (versus echocardiographic assessment) for identifying the low-risk subgroup lacks clarity.12,13 Moreover, studies have not formally evaluated whether the combination of a modified (i.e., heart rate cutoff of 100beats/min) sPESI with CT-assessed RV/left ventricle (LV) ratio might improve the identification of low-risk PE patients. Therefore, this study used the data from the Registro Informatizado de la Enfermedad TromboEmbólica (RIETE), a large ongoing, multi-center, multinational, prospective registry of consecutive patients with objectively confirmed, acute venous thromboembolism (VTE)14–16: (1) to assess the usefulness of a modified (i.e., heart rate cutoff of 100beats/min) sPESI to identify low-risk patients with acute symptomatic PE and (2) to evaluate if the addition of CT-assessed RV/LV ratio to the original and modified sPESI improved the identification of low-risk PE.
MethodsDesign and Data SourcePrevious publications have described the design and conduct of the RIETE registry (ClinicalTrials.gov identifier, NCT02832245).14 Briefly, at each participating RIETE site, investigators aimed to enroll consecutive patients who had acute objectively confirmed VTE. Confirmatory testing for PE consisted of high probability ventilation–perfusion (V/Q) scintigraphy,17 positive contrast-enhanced, PE-protocol, CT for PE,18 or lower limb venous compression ultrasonography positive for proximal deep vein thrombosis (DVT) in patients presenting with PE signs or symptoms.19 All patients provided informed consent for participation in the registry in accordance with local ethics committee requirements.
Patients and EligibilityThis study included patients who were enrolled in RIETE and had a diagnosis of acute symptomatic PE from January 1, 2001, through March 31, 2022. Patients with hemodynamic instability at presentation (i.e., systolic blood pressure <90mmHg), and those in whom the RV/LV ratio on CT could not be measured were excluded.
Calculation of sPESI and Modified sPESIThe sPESI score included the variables of age >80 years, history of cancer, history of chronic cardiopulmonary disease, heart rate ≥110beats/min, systolic blood pressure <100mmHg, and arterial oxygen saturation <90% at the time of diagnosis.6
For this study, we also employed a modified sPESI that used a cutoff ≥100beats/min for heart rate. The original and the modified sPESI categorized patients without any variable present as low-risk, and those with any of the variables present as high-risk.
Computed TomographyLocal radiologists measured the ratio of the right-to-left ventricular short axis diameters. For this study, we defined CT-assessed RV enlargement as a ratio of the RV to the LV short-axis diameters of greater than 1.20
OutcomesThis study used all-cause mortality through 30 days after initiation of treatment as the primary outcome. The secondary outcome was 30-day PE-related mortality. The RIETE investigators used medical record review to assess vital status. For patients who died, further medical record review, and proxy interviews, when necessary, and review of autopsy reports if available, assisted with determination of the date and cause of death. For deaths confirmed by autopsy or those following a clinically severe PE, either after initial PE or shortly after an objectively confirmed recurrent event, in the absence of any alternative diagnosis, the investigators were instructed to judge death as due to fatal PE.
Statistical AnalysesTo compare categorical variables, the analyses used chi-squared or Fisher's exact tests. Continuous data were tested for a normal distribution with the Kolmogorov–Smirnov test. Continuous data that did not follow a normal distribution were compared with the Mann–Whitney U test.
We calculated the proportion of patients designated as low-risk based on the sPESI, the modified sPESI, and the combination of the original and modified sPESI with CT-assessed RV/LV ratio. For the combination strategies, both tests had to be negative to classify patients as low-risk. We then determined the proportion of patients with 30-day death (all-cause mortality and PE-related mortality) among low-risk patients. To assess test prognostic characteristics for predicting all-cause and PE-related mortality, we estimated sensitivity, specificity, and positive and negative predictive values and likelihood ratios for each score. Sensitivity meant the percentage of patients who correctly tested positive with a certain strategy over those who died, while specificity meant the percentage of patients who correctly tested negative with a certain strategy over those who survived. Using the sPESI score as the reference, pairwise comparisons of the proportion of all patients who had low-risk PE, and sensitivity were performed using exact binomial testing, and 95% confidence intervals (CIs) for the absolute differences were calculated using the Agresti and Min approach.21
The prognostic relevance of each strategy as well as single predictors with regard to study outcomes was tested using univariable logistic regression analysis and presented as odds ratios with the corresponding 95% CIs. Potential candidate variables were selected a priori based on their biological plausibility at explaining risk for mortality. Specifically, we considered the following covariates: male gender, immobilization, syncope, myocardial injury, and renal insufficiency. Additionally, we constructed multivariable regression models for each strategy separately. For the manual backward stepwise multivariable logistic regression models, we assessed variables that had a significance level of P less than 0.1 in univariable analyses. Since the proportions of missing data (for covariates) were below 5% and it is implausible that certain patient groups specifically were lost to follow-up (i.e., missing completely at random), we analyzed observed data (complete case analysis) ignoring the missing data.
Comparisons were considered significant if the two-sided P-values were less than 0.05. All statistical analyses were performed with the use of the SPSS/PC software package (version 26, SPSS) and the DTComPair package in R version 3.2.3.
ResultsStudy SampleWe identified 52,979 patients with objectively confirmed symptomatic PE enrolled in RIETE during the study period. After exclusion of patients who had a diagnosis of hemodynamically unstable acute PE (n=1741), and those who did not have the RV/LV ratio measured on CT scan (n=48,121), the study cohort consisted of 3117 normotensive patients with confirmed PE. Patients who had the RV/LV ratio measurements reported in RIETE on CT scan did not differ significantly from those who had not in preexisting medical conditions, and in relevant clinical, physiologic and laboratory parameters (Table S1 in Supplementary Appendix).
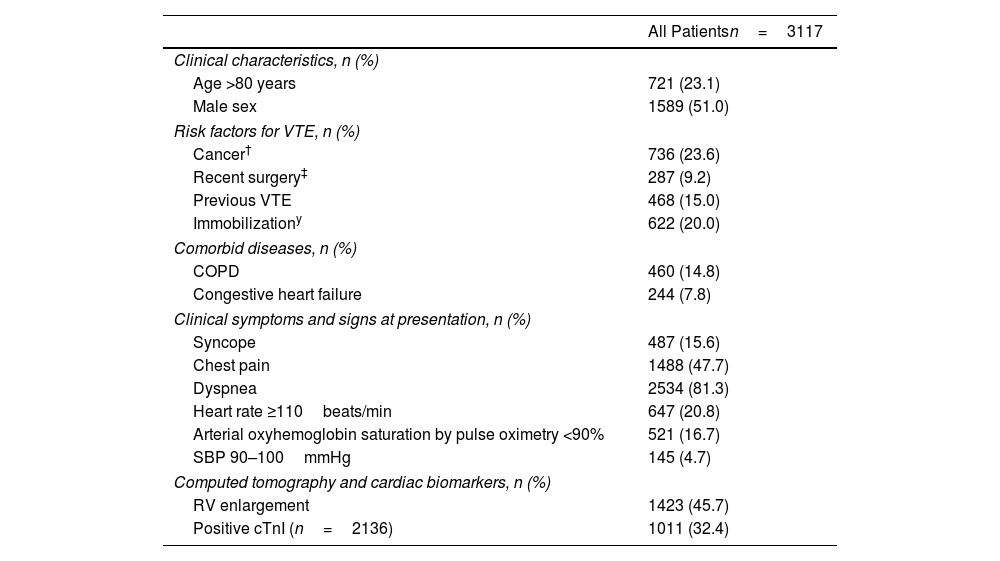
Table 1 shows the patients’ clinical symptoms, predisposing conditions, and relevant findings at presentation. Overall, the mean (SD) age was 67.8 (16.1) years, and 49% of the patients were women. The overall number of patients treated on an outpatient basis (i.e., discharged in the first 24h after diagnosis) was small (13.6%; 424 of 3117 patients).
Baseline Characteristics for 3117 Hemodynamically Stable Patients With Acute Symptomatic Pulmonary Embolism.
| All Patientsn=3117 | |
|---|---|
| Clinical characteristics, n (%) | |
| Age >80 years | 721 (23.1) |
| Male sex | 1589 (51.0) |
| Risk factors for VTE, n (%) | |
| Cancer† | 736 (23.6) |
| Recent surgery‡ | 287 (9.2) |
| Previous VTE | 468 (15.0) |
| Immobilizationy | 622 (20.0) |
| Comorbid diseases, n (%) | |
| COPD | 460 (14.8) |
| Congestive heart failure | 244 (7.8) |
| Clinical symptoms and signs at presentation, n (%) | |
| Syncope | 487 (15.6) |
| Chest pain | 1488 (47.7) |
| Dyspnea | 2534 (81.3) |
| Heart rate ≥110beats/min | 647 (20.8) |
| Arterial oxyhemoglobin saturation by pulse oximetry <90% | 521 (16.7) |
| SBP 90–100mmHg | 145 (4.7) |
| Computed tomography and cardiac biomarkers, n (%) | |
| RV enlargement | 1423 (45.7) |
| Positive cTnI (n=2136) | 1011 (32.4) |
Abbreviations: VTE, venous thromboembolism; COPD, chronic obstructive pulmonary disease; SBP, systolic blood pressure; RV, right ventricle; cTnI, cardiac troponin I.
The study had complete survival information for all patients at the end of the 30-day follow-up period. Regarding the primary outcome, 137 of the 3117 (4.4%; 95% CI, 3.7% to 5.2%) patients died during the 30-day follow-up period. Forty-one patients (1.3%; 95% CI, 1.0% to 1.8%) died from PE, and 96 (3.1%; 95% CI, 2.5% to 3.8%) died from other causes (cancer 35, infection 18, hemorrhage 9, heart failure 7, unknown 10, other 17).
Symptomatic recurrent VTE was objectively confirmed in 15 patients in the cohort (15 of 3117 patients; 0.5%; 95% CI, 0.3% to 0.8%). Ten (0.3%; 95% CI, 0.2% to 0.6%) patients had recurrent symptomatic PE (with or without DVT) and 5 (0.2%; 95% CI, 0.1% to 0.4%) had symptomatic isolated DVT. Major bleeding was objectively confirmed in 113 patients in the cohort (113 of 3117 patients; 3.6%; 95% CI, 3.0% to 4.3%).
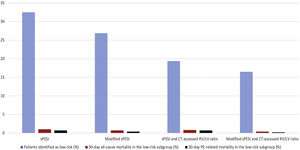
Identification of Low-risk PEUsing patients in this study cohort, the sPESI classified a significantly higher proportion of patients as low-risk (32.5% [1012/3117]; 95% CI, 30.8% to 34.1%) than the modified sPESI (26.9% [839/3117]; 95% CI, 25.4% to 28.5%) (P<0.001), the combination of the original sPESI with CT-assessed RV/LV ratio (19.4% [606/3117]; 95% CI, 18.1% to 20.9%) (P<0.001), or the combination of the modified sPESI with CT-assessed RV/LV ratio (16.5% [514/3117]; 95% CI, 15.2% to 17.8%) (P<0.001) (Fig. 1).
Proportion of patients categorized as having low-risk and failure rates for mortality in the low-risk subgroup according to different prognostic strategies.
Abbreviations: sPESI, simplified Pulmonary Embolism Severity Index; CT, computed tomography; RV, right ventricle; LV, left ventricle; PE, pulmonary embolism.
Of 1012 patients with a sPESI of 0 points, 10 (1.0%, 95% CI, 0.5% to 1.8%) died during the 30-day follow-up. Of 839 (26.9%) patients with a modified sPESI of 0 points, 6 (0.7%; 95% CI, 0.3% to 1.6%) died during the 30-day follow-up (Fig. 1). Six hundred and six (19.4%) patients had a sPESI of 0 points and a CT-assessed RV/LV ratio ≤1, of whom 5 (0.8%; 95% CI, 0.3% to 1.9%) died during the 30-day follow-up. Five hundred and fourteen (16.5%) patients had a modified sPESI of 0 points and a negative CT for RV enlargement, of whom 2 (0.4%; 95% CI, 0.1% to 1.4%) died during the 30-day follow-up. Thirty-day PE-related mortality rates were 0.7%, 0.4%, 0.7%, and 0.2% among low-risk patients identified by the sPESI, modified sPESI, the combination of sPESI and CT-assessed RV/LV ratio, or the combination of modified sPESI and CT-assessed RV/LV ratio, respectively (Fig. 1).
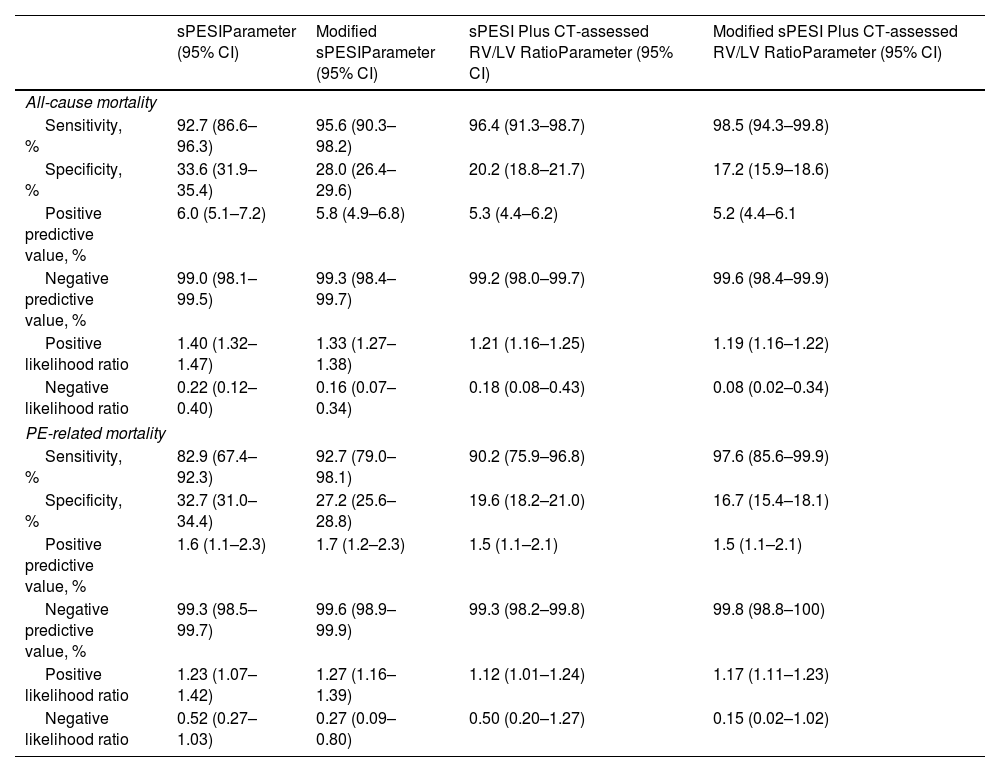
Compared with the sPESI (sensitivity 92.7%), the modified sPESI (sensitivity 95.6%; difference 2.9%, 95% CI, −3.5% to 9.5%; P=0.44), and the combination of the sPESI (sensitivity 96.4%; difference 3.7%, 95% CI, −2.6% to 10.1%; P=0.29) or the modified sPESI with a CT-assessed RV/LV ratio ≤1 (sensitivity 98.5%; difference 5.8%, 95% CI, 0.3% to 12.0%; P=0.03) had higher sensitivities and negative predictive values for predicting all-cause mortality (Table 2). Since these models were designed for identification of low-risk PE patients, specificities, and positive predictive values for predicting mortality were low (Table 2).
Test Characteristics for Identification of 30-Day All-cause and PE-related Mortality.
| sPESIParameter (95% CI) | Modified sPESIParameter (95% CI) | sPESI Plus CT-assessed RV/LV RatioParameter (95% CI) | Modified sPESI Plus CT-assessed RV/LV RatioParameter (95% CI) | |
|---|---|---|---|---|
| All-cause mortality | ||||
| Sensitivity, % | 92.7 (86.6–96.3) | 95.6 (90.3–98.2) | 96.4 (91.3–98.7) | 98.5 (94.3–99.8) |
| Specificity, % | 33.6 (31.9–35.4) | 28.0 (26.4–29.6) | 20.2 (18.8–21.7) | 17.2 (15.9–18.6) |
| Positive predictive value, % | 6.0 (5.1–7.2) | 5.8 (4.9–6.8) | 5.3 (4.4–6.2) | 5.2 (4.4–6.1 |
| Negative predictive value, % | 99.0 (98.1–99.5) | 99.3 (98.4–99.7) | 99.2 (98.0–99.7) | 99.6 (98.4–99.9) |
| Positive likelihood ratio | 1.40 (1.32–1.47) | 1.33 (1.27–1.38) | 1.21 (1.16–1.25) | 1.19 (1.16–1.22) |
| Negative likelihood ratio | 0.22 (0.12–0.40) | 0.16 (0.07–0.34) | 0.18 (0.08–0.43) | 0.08 (0.02–0.34) |
| PE-related mortality | ||||
| Sensitivity, % | 82.9 (67.4–92.3) | 92.7 (79.0–98.1) | 90.2 (75.9–96.8) | 97.6 (85.6–99.9) |
| Specificity, % | 32.7 (31.0–34.4) | 27.2 (25.6–28.8) | 19.6 (18.2–21.0) | 16.7 (15.4–18.1) |
| Positive predictive value, % | 1.6 (1.1–2.3) | 1.7 (1.2–2.3) | 1.5 (1.1–2.1) | 1.5 (1.1–2.1) |
| Negative predictive value, % | 99.3 (98.5–99.7) | 99.6 (98.9–99.9) | 99.3 (98.2–99.8) | 99.8 (98.8–100) |
| Positive likelihood ratio | 1.23 (1.07–1.42) | 1.27 (1.16–1.39) | 1.12 (1.01–1.24) | 1.17 (1.11–1.23) |
| Negative likelihood ratio | 0.52 (0.27–1.03) | 0.27 (0.09–0.80) | 0.50 (0.20–1.27) | 0.15 (0.02–1.02) |
Abbreviations: sPESI, simplified Pulmonary Embolism Severity Index; CT, computed tomography; RV, right ventricle; LV, left ventricle; CI, confidence interval.
Sensitivity: percentage of patients who correctly test positive over those who die. Specificity: percentage of patients who correctly test negative over those who survive. Positive predictive value: percentage of patients who die over those who test positive. Negative predictive value: percentage of patients who survive over those who test negative.
Positive likelihood ratio: change in the odds of dying in patients with a positive test. Negative likelihood ratio: change in the odds of dying in patients with a negative test.
For predicting 30-day PE-related mortality, compared with the sPESI (sensitivity 82.9%), the modified sPESI (sensitivity 92.7%; difference 9.8%, 95% CI, −6.8% to 26.2%; P=0.31), and the combination of the sPESI (sensitivity 90.2%; difference 7.3%, 95% CI, −9.8% to 24.2%; P=0.52) or the modified sPESI with a CT-assessed RV/LV ratio ≤1 (sensitivity 97.6%; difference 14.6%, 95% CI, 0% to 30.4%; P=0.05) had higher sensitivities and negative predictive values (Table 2).
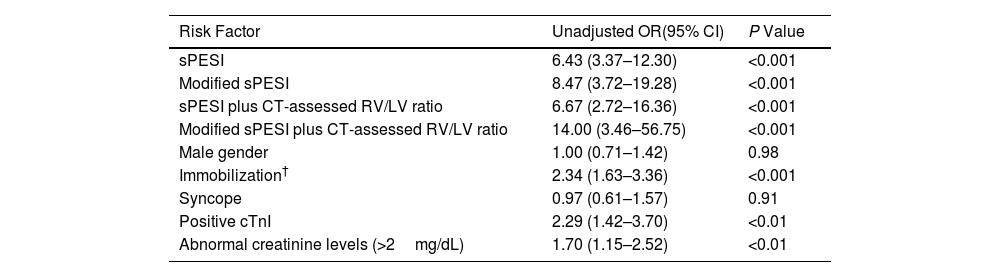
In univariable analysis, all models were associated with a significantly increased risk for 30-day all-cause mortality (Table 3). The highest odds ratio was observed for the combination of the modified sPESI with CT-assessed RV/LV ratio. Additionally, as shown in Table 3, a number of variables were identified as predictors of mortality using univariable logistic regression analysis. After adjustment, the 4 models remained independent predictors of 30-day all-cause mortality (Table 3).
Unadjusted and Adjusted Odds Ratios for Overall Mortality in Patients With Acute Symptomatic Pulmonary Embolism.
| Risk Factor | Unadjusted OR(95% CI) | P Value |
|---|---|---|
| sPESI | 6.43 (3.37–12.30) | <0.001 |
| Modified sPESI | 8.47 (3.72–19.28) | <0.001 |
| sPESI plus CT-assessed RV/LV ratio | 6.67 (2.72–16.36) | <0.001 |
| Modified sPESI plus CT-assessed RV/LV ratio | 14.00 (3.46–56.75) | <0.001 |
| Male gender | 1.00 (0.71–1.42) | 0.98 |
| Immobilization† | 2.34 (1.63–3.36) | <0.001 |
| Syncope | 0.97 (0.61–1.57) | 0.91 |
| Positive cTnI | 2.29 (1.42–3.70) | <0.01 |
| Abnormal creatinine levels (>2mg/dL) | 1.70 (1.15–2.52) | <0.01 |
| Strategy* | Adjusted OR**(95% CI) | P Value |
|---|---|---|
| sPESI | 4.04 (1.84–8.90) | <0.01 |
| Modified sPESI | 5.51 (1.99–15.25) | <0.01 |
| sPESI plus CT-assessed RV/LV ratio | 4.37 (1.35–14.12) | 0.01 |
| Modified sPESI plus CT-assessed RV/LV ratio | 10.88 (1.50–79.14) | 0.02 |
Abbreviations: OR, odds ratio; CI, confidence interval; sPESI, simplified Pulmonary Embolism Severity Index; CT, computed tomography; RV, right ventricle; LV, left ventricle.
Since hypoxemic patients with acute PE might not qualify for outpatient therapy of their disease, we recalculated sensitivity for each strategy after exclusion of patients with oxygen saturation <90%. Compared with the sPESI (sensitivity 90.6%), the modified sPESI (sensitivity 94.3%), and the combination of the sPESI with a CT-assessed RV/LV ratio ≤1 (sensitivity 95.3%) the combination of the modified sPESI with a CT-assessed RV/LV ratio ≤1 showed the highest sensitivity (98.1%) for predicting all-cause mortality.
DiscussionThis study provides validation of different strategies for identifying patients with low-risk acute symptomatic PE. Compared with the sPESI, the combination of a modified sPESI (i.e., heart rate cutoff of 100beats/min) and CT-assessed RV/LV ratio designated a smaller proportion of patients as having ‘low-risk’ PE. However, a combination of a modified sPESI and CT-assessed RV/LV showed a significantly greater ability to predict all-cause and PE-related mortality, compared with the original sPESI. In other words, negative results for both modified sPESI and CT-assessed RV/LV ratio identify a subpopulation of patients with PE who are at a ‘very low-risk’ for 30-day mortality.
Use of the sPESI or the Hestia criteria for identifying low-risk patients with acute symptomatic PE has been extensively validated,22,23 and clinical practice guidelines recommend that risk assessment of stable PE patients should begin with computation of any of these scores.24 However, the utilization of outpatient PE management in clinical practice is relatively low.25 Recently, the ESC guidelines suggested the incorporation of RV enlargement/dysfunction assessment into the clinical scores to drive the decision to treat at home in low-risk stable patients with acute PE.10 Prior studies have shown conflicting data regarding whether RV assessment improves risk stratification in these patients, and how to perform such evaluation (i.e., CT scan versus echocardiography versus cardiac biomarkers).9,11,12,26 Information on CT-assessed RV enlargement is available for most patients at the time of PE diagnosis. In this study, we assessed if the addition of CT to the sPESI improved the identification of low-risk patients with PE. In fact, our results suggest that the absence of RV enlargement on CT improved the prognostic ability of sPESI for identification of patients at very low-risk for mortality. Our study's large sample size and the robustness of the findings provide strong evidence supporting the concept that the combination of a clinical score (i.e., a modified sPESI) and CT-assessed RV/LV ratio accurately identifies short-term all-cause and PE-related death.
The outcomes assessed by prognostic tools for patients with acute PE should have a relationship to the therapeutic options, and clinicians might use alternative cutoffs for identification of low-risk (i.e., more sensitive) versus intermediate-risk (i.e., more specific) patients with acute symptomatic PE.13 Our results support this concept, and the modification of the HR cutoff in the sPESI improves identification of patients with acute PE who have a very low-risk for short-term all-cause mortality.
These analyses might have practical implications. Based on our analysis, PE patients deemed at very low-risk on the basis of a negative modified sPESI and absence of CT-assessed RV enlargement might safely undergo outpatient therapy of their disease. Alternatively, those with RV enlargement and/or borderline tachycardia might benefit from initial hospital observation to ascertain stability prior to home discharge. In this latter subgroup, previous studies have suggested that normalization of heart rate within 48h of initiation of therapy might identify those who benefit from an abbreviated hospital stay without compromising safety.27 Anyway, clinicians may use this information, in conjunction with patient preferences, to determine the appropriate level of care.
Strengths of this study included enrollment of a large cohort of consecutive stable patients with symptomatic, objectively confirmed acute PE, and detailed prospective data collection on all patients. In addition, we used an easy and reproducible parameter to estimate RV enlargement. The strategy can be readily assessed at the time of patient presentation without incurring extra costs of requiring additional resources, and can help physicians determine when outpatient care is appropriate for patients with acute symptomatic PE.
The limitations of this study should be kept in mind for appropriate interpretation and the development of future studies. Though the variables for the clinical decision rule were collected prospectively, this study was a retrospective analysis. One weakness of the study included the restriction of recruitment to patients who had the RV/LV ratio measured on CT. Since the ineligible patients had an even distribution of disease severity and they did not differ significantly from the eligible cohort with respect to baseline characteristics, it is not apparent whether this affected the results. Also, the focus of this study was not on some other factors that can affect decision-making for early discharge, such as bleeding risk with anticoagulation, or ability to comply with outpatient therapy and follow-up. Such issues should be investigated in future studies.
In conclusion, the combination of a negative modified sPESI with CT-assessed RV/LV ratio ≤1 identifies patients with acute PE who are at very low-risk for short-term mortality. Our study can help clinicians on decision-making concerning patient disposition and drive further studies on outpatient therapy.
Authors’ ContributionsStudy concept and design: Jimenez, Bikdeli, Monreal
Acquisition of data; analysis and interpretation of data; statistical analysis: Jimenez, Bikdeli, Rodriguez, Muriel, Ballaz, Soler, Schellong, Gil-Díaz, Skride, Riera-Mestre, Monreal
Drafting of the manuscript: Jimenez, Bikdeli, Rodriguez, Monreal
Study supervision: Jiménez, Monreal
The corresponding author, David Jiménez, had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of InterestD.J. has nothing to disclose.
B.B. reports that he is a consulting expert, on behalf of the plaintiff, for litigation related to two specific brand models of IVC filters.
C.R. has nothing to disclose.
A.M. has nothing to disclose.
A.B. has nothing to disclose.
S.S. has nothing to disclose.
S.S. has nothing to disclose.
A.G.-D. has nothing to disclose.
A.S. has nothing to disclose.
A.R.-M. has nothing to disclose.
M.M. has nothing to disclose.
We express our gratitude to Sanofi Spain, LEO PHARMA and ROVI for supporting this Registry with an unrestricted educational grant. We also thank the RIETE Registry Coordinating Center, S&H Medical Science Service, for their quality control data, logistic and administrative support.
Dr. Bikdeli is supported by the Scott Schoen and Nancy Adams IGNITE Award from the Mary Horrigan Connors Center for Women's Health and Gender Biology at Brigham and Women's Hospital and a Career Development Award from the American Heart Association.
Coordinator of the RIETE Registry: Manuel Monreal.
RIETE Steering Committee Members: Paolo Prandoni, Benjamin Brenner and Dominique FArge-Bancel.
Raquel Barba (Spain), Pierpaolo Di Micco (Italy), Laurent Bertoletti (France), Sebastian Schellong (Germany), Inna Tzoran (Israel), Abilio Reis (Portugal), Marijan Bosevski (R. Macedonia), Henri Bounameaux (Switzerland), Radovan Malý (Czech Republic), Peter Verhamme (Belgium), Joseph A. Caprini (USA), Hanh My Bui (Vietnam).
RIETE Registry Coordinating Center: S&H Medical Science Service.
SPAIN: Adarraga MD, Amado C, Arcelus JI, Ballaz A, Barba R, Barbagelata C, Barrón M, Barrón-Andrés B, Blanco-Molina A, Beddar Chaib F, Botella E, Cañas I, Casado I, Chasco L, Criado J, de Ancos C, de Miguel J, del Toro J, Demelo-Rodríguez P, Díaz-Brasero AM, Díaz-Pedroche MC, Díaz-Peromingo JA, Domínguez IM, Dubois-Silva A, Escribano JC, Espósito F, Farfán-Sedano AI, Falgá C, Fernández-Capitán C, Fernández-Jiménez B, Fernández-Muixi J, Fernández-Reyes JL, Fernández V, Font C, Francisco I, Galeano-Valle F, García MA, García-Bragado F, García de Herreros M, Gavín-Blanco O, Gil-Díaz A, Gómez-Cuervo C, Gómez-Mosquera AM, Grau E, Guirado L, Gutiérrez J, Hernández-Blasco L, Jara-Palomares L, Jaras MJ, Jiménez D, Jou I, Joya MD, Lacruz B, Lainez-Justo S, Lalueza A, Latorre A, Lima J, Lobo JL, López-Brull H, López-De la Fuente M, López-Jiménez L, López-Meseguer M, López-Miguel P, López-Núñez JJ, López-Reyes R, López-Sáez JB, Lorente MA, Lorenzo A, Madridano O, Maestre A, Marchena PJ, Martín-Guerra JM, Martín-Martos F, Martínez-Redondo I, Mellado M, Mena E, Moisés J, Mercado MI, Monreal M, Muñoz-Blanco A, Muñoz-Gamito G, Morales MV, Nieto JA, Núñez-Fernández MJ, Olid-Velilla M, Osorio J, Otalora S, Otero R, Paredes-Ruiz D, Parra P, Parra V, Pedrajas JM, Peris ML, Pesce ML, Porras JA, Poyo-Molina J, Puchades R, Riera-Mestre A, Rivera-Civico F, Rivera-Gallego A, Roca M, Rosa V, Rodríguez-Cobo A, Rodríguez-Matute C, Ruiz-Artacho P, Ruiz-Giménez N, Ruiz-Ruiz J, Salgueiro G, Sancho T, Sendín V, Sigüenza P, Soler S, Suárez-Rodríguez B, Suriñach JM, Tiberio G, Torres MI, Torres-Sánchez A, Trujillo-Santos J, Uresandi F, Usandizaga E, Valle R, Varona JF, Vela L, Vela JR, Villalobos A, Villares P; AUSTRIA: Ay C, Nopp S, Pabinger I; BELGIUM: Engelen MM, Vanassche T, Verhamme P; BRAZIL: Yoo HHB; COLOMBIA: Arguello JD, Montenegro AC, Roa J; CZECH REPUBLIC: Hirmerova J, Malý R, Varhaník F; FRANCE: Accassat S, Bertoletti L, Bura-Riviere A, Catella J, Chopard R, Couturaud F, Espitia O, El Harake S, Falvo N, Le Mao R, Mahé I, Moustafa F, Plaisance L, Sarlon-Bartoli G, Suchon P, Versini E; GERMANY: Schellong S; ISRAEL: Braester A, Brenner B, Kenet G, Tzoran I; IRAN: Sadeghipour P; ITALY: Basaglia M, Bilora F, Bortoluzzi C, Brandolin B, Colaizzo D, Ciammaichella M, De Angelis A, Dentali F, Di Micco P, Grandone E, Imbalzano E, Merla S, Pesavento R, Prandoni P, Siniscalchi C, Tufano A, Visonà A, Vo Hong N, Zalunardo B; LATVIA: Dzirnieks K, Gibietis V, Skride A; PORTUGAL: Fonseca S, Manuel M, Meireles J; REPUBLIC OF MACEDONIA: Bosevski M, Trajkova M, Zdraveska M; SWITZERLAND: Bounameaux H, Fresa M, Keller S, Mazzolai L; UNITED KINGDOM: Aujayeb A; USA: Caprini JA, Weinberg I; VIETNAM: Bui HM.