The respiratory syncytial virus (RSV) causes a substantial burden worldwide. After over six decades of research, there is finally a licensed immunization option that can protect the broad infant population, and other will follow soon. RSV immunization should be in place from season 2023/2024 onwards. Doing so requires thoughtful but swift steps. This paper reflects the view of four immunization experts on the efforts being made across the globe to accommodate the new immunization options and provides recommendations organized around five priorities: (I) documenting the burden of RSV in specific populations; (II) expanding RSV diagnostic capacity in clinical practice; (III) strengthening RSV surveillance; (IV) planning for the new preventive options; (V) achieving immunization targets. Overall, Spain has been a notable example of converting RSV prevention into a national desideratum and has pioneered the inclusion of RSV in some of the regional immunization calendars for infants facing their first RSV season.
Discovered in 1956, the respiratory syncytial virus (RSV) is a common respiratory virus that can lead to severe illness in infants and young children.1,2 Tackling the burden of RSV has for long been a global health priority. However, until recently, the only approved prophylaxis for RSV was a monoclonal antibody (mAb) called palivizumab. This mAb was authorized for use in the European Union (EU) in 1999, for specific groups of children at high-risk of RSV disease, and requires monthly injections to sustain protection.3,4 This left many children unprotected against RSV, as most cases are observed in healthy infants born at term.5–8
After more than six decades of research, there is finally a licensed immunization option that can protect the broad infant population.9,10 In November 2022, nirsevimab was approved in the EU for the prevention of RSV lower respiratory tract infections (LRTI) in neonates and infants born at term or preterm during their first RSV season, with or without specific health conditions, for at least five months.3,9–16 The Food and Drug Administration (FDA) Advisory Committee has also unanimously recommended nirsevimab as first immunization against RSV disease for all infants.17 The implementation of this new preventive strategy is expected to reduce the burden of RSV-related LRTI, namely medical attention and hospitalization due to RSV as well as of medically attended LRTI of any cause.3,11,14,18–22
Other active and passive immunization options, including vaccines, are in late-stage clinical development.3,9 The FDA has recently approved two RSV vaccines for adults over 60 years old and its Advisory Committee has voted in favour of the approval of a maternal RSV vaccine to protect infants from severe RSV disease during the first 6 months of life.23–27
Three regions in Spain (Galicia – first place in the world to officially announce it, Catalonia and Madrid) have already confirmed the start of nirsevimab prophylaxis in all infants for the season 2023–2024. This paper reflects the view of four Spanish immunization experts on the rational of these decisions and on the efforts being made across the globe to accommodate the new immunization options by 2023. It addresses the need for an improved RSV surveillance system and detailed data on disease burden to assist policy makers in the implementation of an RSV immunization preventive strategy. The review is conducted at a global level but provides more in-depth insights for Spain. Recommendations cover primary care (PC), hospital care, and public health perspectives.
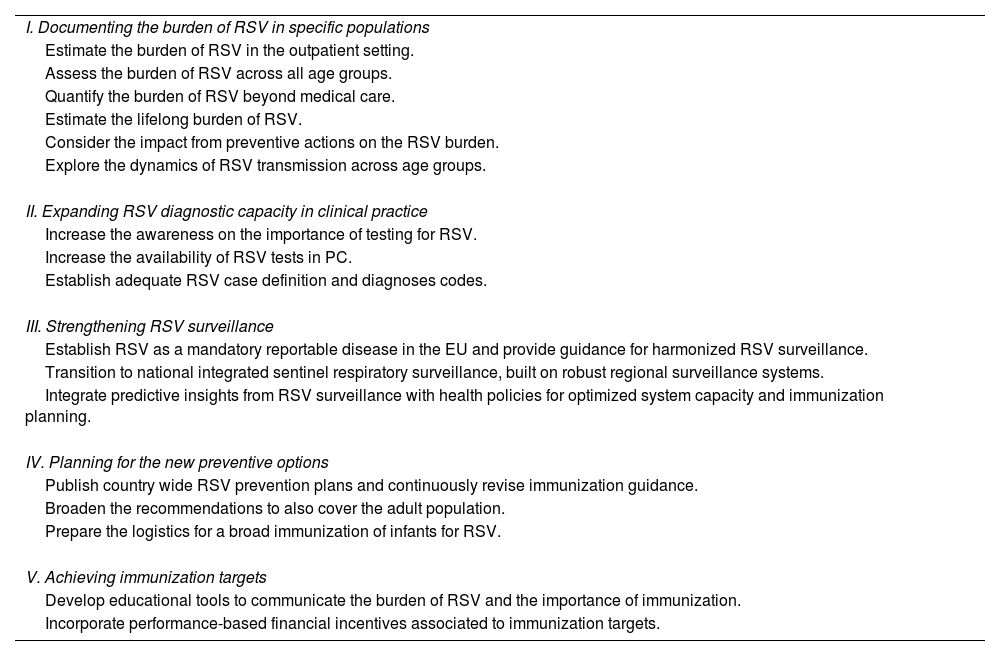
Recommended actionsTable 1 summarizes our recommendations, organized around five areas: (I) documenting the burden of RSV in specific populations; (II) expanding RSV diagnostic capacity in clinical practice; (III) strengthening RSV surveillance; (IV) planning for the new preventive options; (V) achieving immunization targets.
- I.
Documenting the burden of RSV in specific populations
List of recommended actions.
| I. Documenting the burden of RSV in specific populations |
| Estimate the burden of RSV in the outpatient setting. |
| Assess the burden of RSV across all age groups. |
| Quantify the burden of RSV beyond medical care. |
| Estimate the lifelong burden of RSV. |
| Consider the impact from preventive actions on the RSV burden. |
| Explore the dynamics of RSV transmission across age groups. |
| II. Expanding RSV diagnostic capacity in clinical practice |
| Increase the awareness on the importance of testing for RSV. |
| Increase the availability of RSV tests in PC. |
| Establish adequate RSV case definition and diagnoses codes. |
| III. Strengthening RSV surveillance |
| Establish RSV as a mandatory reportable disease in the EU and provide guidance for harmonized RSV surveillance. |
| Transition to national integrated sentinel respiratory surveillance, built on robust regional surveillance systems. |
| Integrate predictive insights from RSV surveillance with health policies for optimized system capacity and immunization planning. |
| IV. Planning for the new preventive options |
| Publish country wide RSV prevention plans and continuously revise immunization guidance. |
| Broaden the recommendations to also cover the adult population. |
| Prepare the logistics for a broad immunization of infants for RSV. |
| V. Achieving immunization targets |
| Develop educational tools to communicate the burden of RSV and the importance of immunization. |
| Incorporate performance-based financial incentives associated to immunization targets. |
EU, European Union; PC, primary care; RSV, respiratory syncytial virus.
Having detailed data on disease burden is essential to inform resource allocation decisions for immunization policies.10 Every day, new studies are being published on the burden of RSV.5,28–31 Spain is not an exception. In the last two years, novelty data has been published, addressing some of the existing evidence gaps.5,7,32–44 We identify six areas warranting further research.
- 1.
Estimate the burden of RSV in the outpatient and emergency room setting
While the burden of RSV hospitalizations is well characterized, it only covers the most severe cases. Furthermore, cases are often coded according to the clinical manifestations and complications from RSV conducting to hospitalisations and not recorded as associated to RSV due to lack of testing.45,46 Not accounting for RSV episodes managed in the outpatient setting results in an underestimated disease burden as most bronchiolitis cases are managed in PC.47 Recent studies report that, amongst children aged<2 years old with RSV in Spain, 95–97% visited the PC, 58–62% visited the emergency department, 28–35% were hospitalized, and at least 2% were admitted to intensive care units.7,38 More studies are required to fully grasp the clinical and economic burden of RSV beyond hospitalizations. Initiatives such as the RSV Hospital Emergency Departments in Iberia (RHEDI) surveillance study, built to capture the RSV burden in infants attending emergency services in the Iberian Peninsula, and the RSV ComNet, aimed to measure the clinical and socio-economic disease burden of RSV in young children in PC across countries, should be further promoted.5,44,48
- 2.
Assess the burden of RSV across all age groups
Studies tend to focus on young children, as it is where most of the burden is found. All infants are at risk of acquiring an RSV infection with severe complications, with most RSV cases being observed in children without underlying medical conditions.6,7,49 However, age plays an important role in the risk of developing LRTI and of being hospitalized due to RSV.50 There is a need for a greater granularity on the patients’ age reported on burden of disease studies to enable more informed decisions on immunization strategies, namely considering the months of life at the beginning of RSV season.50 Since RSV-associated in-hospital mortality was reported to increase with age, we recommend the burden of RSV in the adult population to be further explored as it may be underestimated, particularly in frail and high-risk older adults.31,39,51
- 3.
Quantify the burden of RSV beyond medical care
Indirect costs related to productivity losses make up a significant part of the burden of RSV, even in mild cases. A recent study in the UK estimated that RSV in children under 5 years old generated an annual cost of £80 million, with 17% attributed to productivity losses, 2% to out-of-pocket expenses for parents/carers, and 81% to direct healthcare costs.52
The Respiratory Syncytial Virus Consortium in Europe (RESCEU) study, which enrolled 9164 healthy, term-born infants in five European countries (including Spain) between 2017 and 2020, found that even non-medically attended RSV episodes had a significant impact on infants.5 Respiratory symptoms lasted an average of 14 days in infants causing parents to miss their work duties.5 RSV was also associated with significant reductions in health-related quality of life for up to 14 days after diagnosis, both in children and their parents.38,53,54 Further research is needed to fully understand the impact of RSV on patients and caregivers, including their ability to work or study during RSV episodes.
- 4.
Estimate the lifelong burden of RSV
It is increasingly evident that RSV infection in early childhood is associated with long-term health and economic burden.3,9,55–63 Children with a history of RSV LRTI are estimated to have between two to twelve times greater risk of developing asthma.56,62,63 Moreover, early childhood LRTI was associated with a two-fold risk of premature adult death from respiratory disease.64 Future research is needed to understand the relationship and causality between RSV and chronic lung diseases evolution, and to measure the impact that immunization and preventive strategies may have on these long-term complications.55,60,61
- 5.
Consider the impact from preventive actions on the RSV burden
Healthy, term-born infants account for approximately 98% of RSV cases.65 Yet, one must not forget that children with underlying medical conditions or born preterm are at higher risk of developing severe illness from an RSV infection.5–8 These children tend to have longer hospital stays and worse outcomes.5–8 It is unclear how much the use of palivizumab in at-risk groups may affect study results. As immunization against RSV becomes more prevalent, it's increasingly important for studies on the burden of RSV to incorporate the immunization context of the studied population.
- 6.
Explore the dynamics of RSV transmission across age groups
Dynamic transmission models, paired with cost-effectiveness assessments, are expected to be valuable tools to evaluate and compare the effectiveness of alternative interventions, playing an important role when defining immunization strategies.66 As RSV is a highly contagious pathogen, dynamic transmission models should be developed to explore if the immunization of specific populations has a protective effect on others.10,66–68 Population-based birth cohort studies have shown that the risk of RSV is higher in infants with older siblings, supporting vaccination strategies which include family members to offer an optimal protection for newborn babies.69–73 As an example, vaccination of school-aged children has been shown to diminish influenza infections in the unvaccinated adult population, as well as the number of visits and hospitalizations related to influenza.74–78
- II.
Expanding RSV diagnostic capacity in clinical practice
RSV testing is not yet embedded in clinical practice. This is particularly true in potential RSV cases observed outside the typical RSV season, in population aged above 6 months, and outside of the hospitals. We recommend expanding RSV diagnostic capacity, by increasing the availability of RSV tests, generating awareness on the importance of routine viral testing, and reviewing case definitions. We reckon that paediatricians might resist testing for RSV in PC, due to the prevailing belief that it will not change patient management.32
- 7.
Increase the awareness on the importance of testing for RSV
There are many reasons why one should test for RSV. First, even though it remains disbelieved, knowing which respiratory virus is present enables a better patient management.32,79–81 Understanding the risk of disease progression can help to identify those who require a closer monitorization.32,82 Patients who test positive for RSV have more severe LRTI episodes than those with other respiratory viruses.32,38,47 Second, the viral diagnosis informs the actions to be taken to minimize the risk of transmission, which may be particularly relevant in hospitalized/institutionalized patients as respiratory infections are associated with worse clinical outcomes.32,83 Third, having a viral diagnosis may allow for targeted antiviral treatment, if available, and has been shown to reduce the unnecessary use of antibiotics, which is also a matter of public health concern.32,84,85 This need is more preeminent as there are several RSV-specific antivirals in human clinical trials.86 Fourth, it helps to track epidemiologic trends when data is used by surveillance systems. Understanding the onset and offset of RSV seasons amongst regions is helpful when planning immunization and hospital admission capacity.32,36 The subgroup identification may also aid in healthcare planning, as RSV-A cases are associated with longer hospital stays and higher risk of requiring intensive care and respiratory support than RSV-B.40 Finally, it may provide relevant information for physicians and families on the possible disease course and long-term sequelae, such as the risk of developing wheezing and/or asthma after RSV.32,87 We recommend increasing the awareness on the importance of testing for respiratory virus, such as RSV, rebating the idea that it will not alter the patient management.
- 8.
Increase the availability of RSV tests in PC
To the best of our knowledge, in Spain, most PC centres do not have RSV tests available, with the notable exception of Catalonia. In this region, the incorporation of rapid RSV tests in PC has led to an increase in the average age of reported RSV diagnoses, illustrating the value of routine testing to properly understand the disease burden. When choosing a test, it is important to strike a balance between accessibility, speed and accuracy.32,53 Antigen-based tests are not sensitive enough to be used in older children or adults.88 However, they can still be helpful to detect epidemiologic trends and have proven to be useful for predictive models tested by surveillance systems.89 Given their high sensitivity and specificity, we recommend that molecular-based RSV tests are used, as recommended by the World Health Organization (WHO) and the European Centre for Disease Prevention and Control (ECDC).53,88,90 Furthermore, where possible, all specimens from patients with respiratory symptoms taken from primary and secondary care sentinel surveillance should be tested using multiplex PCR assays to simultaneously detect different relevant respiratory viruses.90
- 9.
Establish adequate RSV case definition and diagnoses codes
RSV surveillance requires more sensitive case definitions to avoid underestimating the disease burden. Definitions may need to vary according to age.91 There is already consensus on using acute respiratory infection (ARI) case definitions for RSV surveillance, instead of influenza-like-illness (ILI) or severe acute respiratory infection (SARI), as having fever as a criteria lowers the sensitivity for RSV case detection in young children.91–95 We strongly advise that other diagnoses are incorporated in RSV surveillance, such as otitis media, which is very frequently observed in infants with RSV infection.96–98 It is also important to revise how RSV is being coded and to establish adequate codification practices. There is evidence that RSV-specific International Classification of Diseases-10 (ICD-10) codes only capture a fraction of true RSV cases.45,46 Furthermore, the International Classification of Primary Care-2 (ICPC-2) does not include any code for RSV.
- III.
Strengthening RSV surveillance
Up until recently, most countries detected RSV infections within existing surveillance systems for influenza.91 With the advent of new immunization solutions, and with the COVID-19 pandemic acting as a catalyst, an international movement is underway to improve RSV surveillance. Anticipating this moment, the WHO has been piloting globally compatible RSV-disease-burden surveillance systems.91,99–101 The goal is to have RSV embedded in national surveillance programmes in 2023, by transitioning to end-to-end integrated sentinel surveillance of influenza, SARS-CoV-2 and RSV.99 Molecular surveillance is essential to monitor the geotemporal evolution of RSV strains and enable early detection of potential escape variants that can impact RSV transmission and the effectiveness of immunization.102,103
- 10.
Establish RSV as a mandatory reportable disease in the EU and providing guidance for harmonized RSV surveillance
Although RSV is not yet a mandatory reportable disease in the European Union/European Economic Area (EU/EEA) level, in 2022, twelve EU/EEA countries had already made RSV a notifiable disease.104 We are in favour of adding RSV to the list of mandatory notifiable diseases at the EU level, to allow EU-wide data analysis. Guidance for a harmonized RSV surveillance within the EU would also be welcomed.
- 11.
Transition to national integrated sentinel respiratory surveillance, built on robust regional surveillance systems
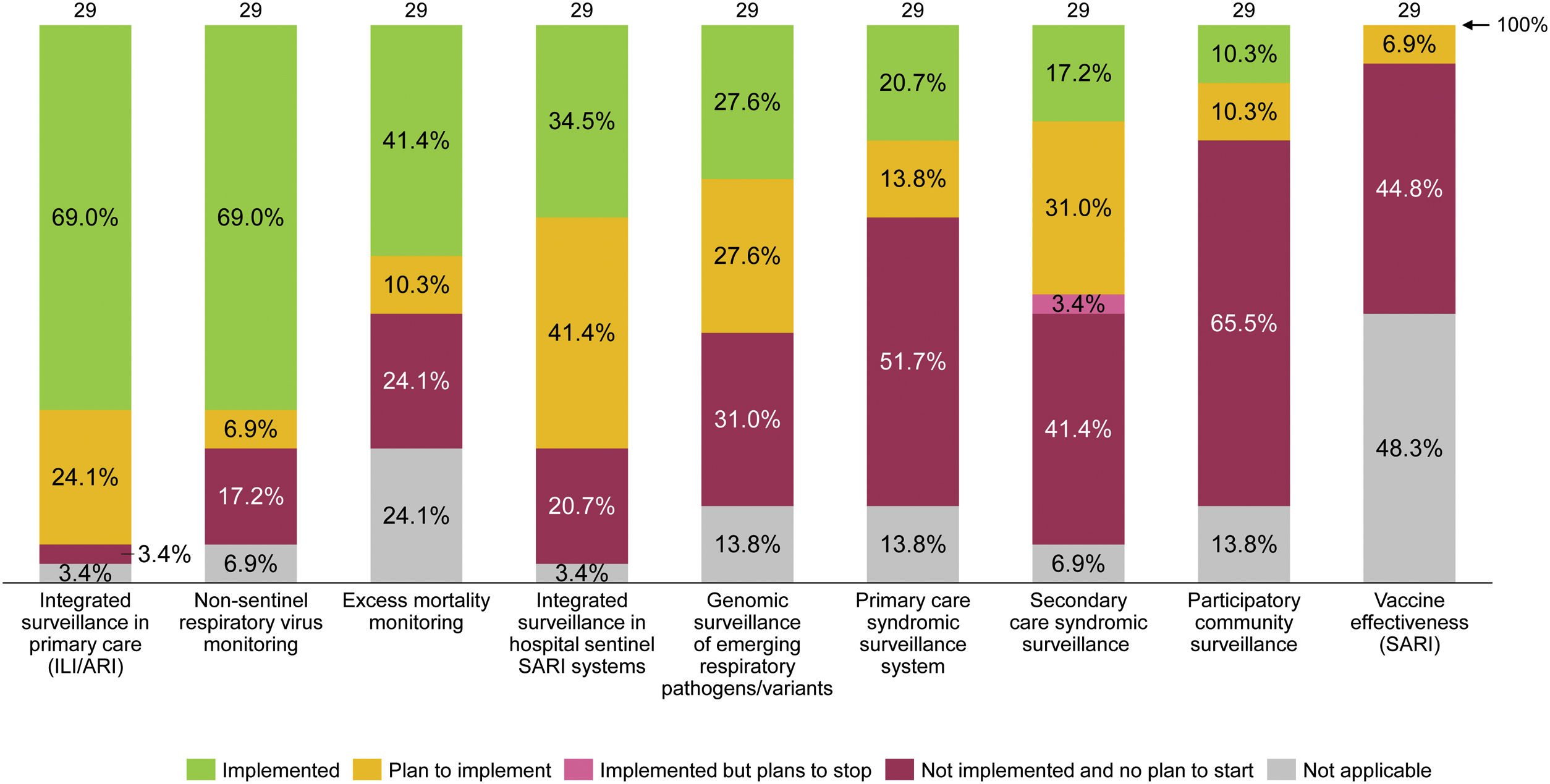
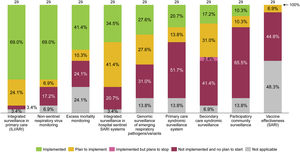
Most of the EU/EEA countries foresee including respiratory viruses other than influenza and SARS-CoV-2, such as RSV, in their surveillance systems.104 In 2022, twelve countries had already started transitioning to integrated sentinel respiratory surveillance, as illustrated in Fig. 1. In Spain, the previous influenza surveillance system was modified in 2021 to incorporate the surveillance of acute respiratory infections – including influenza, COVID-19, and RSV – and is now called Sistema de Vigilancia de la Infección Respiratoria Aguda (SiVIRA). This all-year surveillance will integrate data from PC, hospitals, microbiology, and genomic laboratories. We commend this transition but underline the importance of building the national surveillance system on cohesive and robust regional surveillance systems.
- 12.
Integrate predictive insights from RSV surveillance with health policies related to healthcare system capacity readiness and RSV immunization
Planned characteristics of integrated surveillance for RSV in EU/EEA countries, June 2022. Note: Figure elaborated by the authors with data collected from a survey performed, between 15 March and 9 June 2022, by the ECDC and the WHO to 29 of the 30 EU/EEA countries.104 ARI, acute respiratory infection; ECDC, European Centre for Disease Prevention and Control; EU/EEA, European Union/European Economic Area; ILI, influenza-like illness; SARI, severe acute respiratory infections; WHO, World Health Organization.
In Spain, Catalonia was the first region integrating the functions of epidemiological surveillance of influenza, COVID-19, RSV and other respiratory viruses.105 The developed system presents a set of unique characteristics, detailed in Supplementary Materials (Table S1). A splendid example is the linkage of RSV surveillance data with public health policies. Catalonia intends to achieve better capacity planning by using surveillance data from PC to anticipate RSV hospitalization peaks within a 2–3-week window.89 We see a great added value in this approach, and recommend incorporating similar predictive models in the national surveillance systems. Incorporating near real-time accurate data from different regions would enable a greater anticipation of RSV outbreaks. This could help to prepare spaces for the predicted increased demand for care. It can also facilitate the implementation of immunization strategies.
- IV.
Planning for the new preventive options
No innovation adds value unless it reaches the population that will benefit from it. RSV prevention is rapidly entering the political agendas, with scientific associations and policy makers joining efforts to make immunization available. Experts are reviewing the most adequate preventive option and strategies to each population group, to maximize the impact of immunization in public health. Regulatory bodies need to incorporate these new interventions in their evaluation processes and to provide guidance on how they will be funded and implemented at national and regional level.106
- 13.
Publish country wide RSV prevention plans and continuously revise immunization guidance
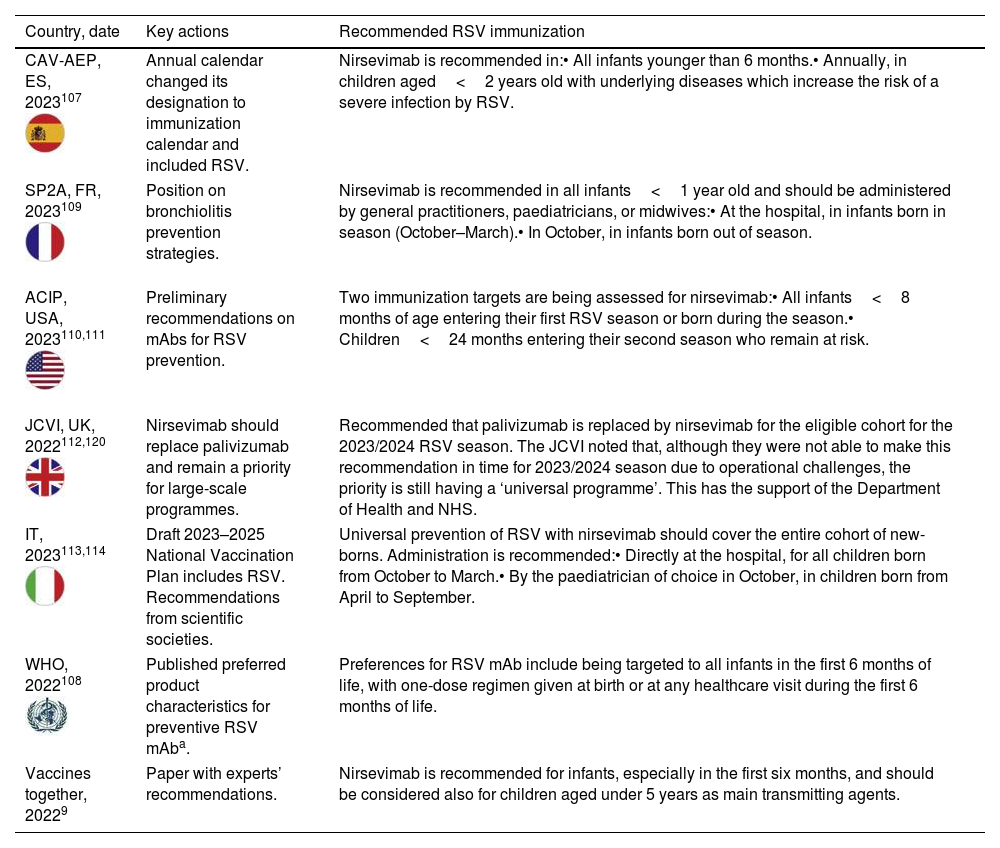
Table 2 summarizes recent updates in RSV prevention plans or immunization recommendations from several countries. We applaud the efforts from the international community and the example set by Spain, who has turned RSV prevention into a national desideratum, and three regions have pioneered the inclusion of RSV in the immunization calendars for infants facing their first RSV season.107
Summary of recent actions related to updated RSV prevention plans or immunization recommendations.
| Country, date | Key actions | Recommended RSV immunization |
|---|---|---|
| CAV-AEP, ES, 2023107 | Annual calendar changed its designation to immunization calendar and included RSV. | Nirsevimab is recommended in:• All infants younger than 6 months.• Annually, in children aged<2 years old with underlying diseases which increase the risk of a severe infection by RSV. |
| SP2A, FR, 2023109 | Position on bronchiolitis prevention strategies. | Nirsevimab is recommended in all infants<1 year old and should be administered by general practitioners, paediatricians, or midwives:• At the hospital, in infants born in season (October–March).• In October, in infants born out of season. |
| ACIP, USA, 2023110,111 | Preliminary recommendations on mAbs for RSV prevention. | Two immunization targets are being assessed for nirsevimab:• All infants<8 months of age entering their first RSV season or born during the season.• Children<24 months entering their second season who remain at risk. |
| JCVI, UK, 2022112,120 | Nirsevimab should replace palivizumab and remain a priority for large-scale programmes. | Recommended that palivizumab is replaced by nirsevimab for the eligible cohort for the 2023/2024 RSV season. The JCVI noted that, although they were not able to make this recommendation in time for 2023/2024 season due to operational challenges, the priority is still having a ‘universal programme’. This has the support of the Department of Health and NHS. |
| IT, 2023113,114 | Draft 2023–2025 National Vaccination Plan includes RSV. Recommendations from scientific societies. | Universal prevention of RSV with nirsevimab should cover the entire cohort of new-borns. Administration is recommended:• Directly at the hospital, for all children born from October to March.• By the paediatrician of choice in October, in children born from April to September. |
| WHO, 2022108 | Published preferred product characteristics for preventive RSV mAba. | Preferences for RSV mAb include being targeted to all infants in the first 6 months of life, with one-dose regimen given at birth or at any healthcare visit during the first 6 months of life. |
| Vaccines together, 20229 | Paper with experts’ recommendations. | Nirsevimab is recommended for infants, especially in the first six months, and should be considered also for children aged under 5 years as main transmitting agents. |
ACIP, Advisory Committee on Immunization Practices; CAV-AEP, Comité Asesor de Vacunas de la Asociación Española de Pediatría; ES, Spain; FR, France; IT, Italy; JCVI, Joint Committee on Vaccination and Immunization; mAb, monoclonal antibody; RSV, respiratory syncytial virus; SP2A, Société pédiatrique de pneumologie et d’allergologie; UK, United Kingdom; USA, Unites States of America; WHO, World Health Organization.
a Published preferred product characteristics for long-acting RSV mAb include being: (i) indicated for the prevention of severe RSV disease during early infancy, as it is the period of highest risk of severe RSV disease and mortality; (ii) targeted to all infants in the first 6 months of life; (iii) administered with a one-dose regimen, that could be given as a birth dose or at any healthcare visit during the first 6 months of life; (iv) comparable with WHO recommended vaccines given at the same age in terms of safety and reactogenicity; (v) effective against RSV-confirmed severe disease for five months following administration (≥70% efficacy); (vi) able to protect against both RSV A and B subtypes; (vii) administered as a single intramuscular or subcutaneous dose using standard volumes for injection; (viii) accessible and affordable to low-and middle-income countries in order to allow broad protection of the most vulnerable infants; amongst other criteria.108
Important moves include the WHO international standards for RSV vaccines and mAb, to harmonize their evaluation, and national guidelines for the RSV immunization, in terms of target population, place and timing of administration, amongst others.9,107–114 The extent to which these recommendations may need to be revised in the future will depend on factors such as the duration of the protection provided by the mAbs or vaccines and its long-term efficacy and safety, but also on the evaluation of potential additional benefits associated to the reduction in the use of antibiotics and of medically attended LRTI of any cause. Results should be continuously assessed to determine the optimal frequency of immunization.
- 14.
Broaden the recommendations to also cover the adult population
We advocate for scientific societies to publish guidance also for the immunization of the adult population. Medical societies treating patients with comorbidities should be involved in the elaboration of these guidelines, but we consider that immunization recommendations will more successful if they are based on age groups instead of specific medical conditions.
- 15.
Prepare the logistics for a broad immunization of infants for RSV
To achieve a broad immunization of infants in time for the RSV season, proper structures must be in place, including processes for the administration and data reporting in all health centres, and sufficient and on-time product stock. Overall, we consider that the logistics will be less challenging in countries such as Spain, who have a good culture of vaccination and a close follow-up of infants through the public PC system. This enables both immunization at the hospital or in one of the programmed follow-up consultations after birth. If a long-lasting effect of RSV immunization is shown in adults, we would advise that the administration is performed throughout the year to reach more people.
- V.
Achieving immunization targets
Finally, we would like to stress the importance of generating awareness on the burden of RSV and benefits of immunization, and of certifying that there are incentives in place that capture the urgency to achieve immunization targets.
- 16.
Develop educational tools to communicate the burden of RSV and the importance of immunization
In Spain, we do not anticipate barriers from paediatricians in the immunization for RSV as there is already a strong culture of vaccination. Challenges are expected to be encountered in general practitioners and other medical specialties, who may be less aware of the impact of RSV, and, in adults, due to the less rooted culture of vaccination and the lack of awareness regarding RSV infection in countries where culture of vaccination is less present for respiratory infections.115 We recommend developing campaigns and educational tools for parents and clinicians focused on spreading the message that RSV affects people of all ages and that risk of infection can, and should, be mitigated. For us, the best spokespersons will be parents of children that were hospitalised with RSV during infancy.116 Patients’ associations have an important role in generating awareness and should be promoted. A good example is the RSV Patient Network founded in 2016 by the RESCEU Patient Advisory Board.117
- 17.
Incorporate performance-based financial incentives associated to immunization targets
Immunization is critical to fight respiratory infections and high coverage rates are likely only feasible with the involvement of PC health care workers (HCW). We recommend incorporating performance-based financial incentives to PC HCW as a policy instrument to increase coverage rates, as performed in other European countries for vaccines against influenza and SARS-CoV-2.118,119
ConclusionsReducing the burden of RSV is a public health priority. Countries should target to have RSV immunization in place from season 2023/2024 onwards. Doing so requires thoughtful but swift steps. This paper aims to contribute to this great effort, by recommending seventeen actions organized around five priority areas.
Authors’ contributionsAll authors have equally contributed to the redaction and revision of the manuscript. The views reflected in this publication are personal and do not necessarily reflect the views of the institutions to which the authors are affiliated.
Ethics approval and consent to participateN/A.
Consent for publicationN/A.
FundingSanofi has funded the medical writing and editorial support of this manuscript. The authors were not paid for writing the publication.
Conflict of interestSanofi has funded the medical writing and editorial support of this manuscript. The authors were not paid for writing the publication.
FMT received honoraria from GSK group of companies, Pfizer Inc, Sanofi Pasteur, MSD, Seqirus, and Janssen for taking part in advisory boards and expert meetings and for acting as a speaker in congresses outside the scope of the submitted work. FMT has also acted as principal investigator in randomized controlled trials of the above-mentioned companies as well as Ablynx, Gilead, Regeneron, Roche, Abbott, Novavax, and MedImmune, with honoraria paid to his institution. AS-A, JANA and MG have nothing to declare regarding this manuscript.
The authors would like to thank Mafalda Carmo (IQVIA, Barcelona, Spain) and Carmen Barrull (IQVIA, Barcelona, Spain) for medical writing and editorial assistance.