Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease, and in some patients, extrapulmonary manifestations can worsen prognosis.1
COPD patients often have other concomitant diseases, particularly cardiovascular conditions, and it has been postulated that COPD plays a significant role in the pathogenesis of these processes.2
Hyponatremia developing during hospitalization for a COPD exacerbation is relatively common, and is associated with a poorer clinical course.3 Low sodium in blood may be a sign of water retention due to other comorbidities, such as heart or kidney failure, drug treatments, adrenal insufficiency after withdrawal of corticosteroids, or syndrome of inappropriate ADH secretion (SIADH). Diseases that may present with SIADH include lung infections (pneumonia, pulmonary abscess, tuberculosis, aspergillosis), asthma, COPD, lung tumors, cystic fibrosis, and acute respiratory failure.4 Hypoxia is associated with ADH secretion,5 but hypercapnia is more commonly associated with this phenomenon.
Both in stable COPD and during exacerbations, hyponatremia, due to its prevalence, its impact on prognosis, and varying etiologies (which can coexist), is a challenge for the clinician and requires appropriate follow-up and treatment. Although the relationship between COPD and SIADH is often mentioned in the literature, we did not find any references to hyponatremia caused by SIADH in COPD (Medline and Pubmed searches, keywords: SIADH and COPD).
For this reason, we believe that our report of a patient with SIADH due to COPD is of interest and provides a good illustration of certain aspects of the differential diagnosis and treatment.
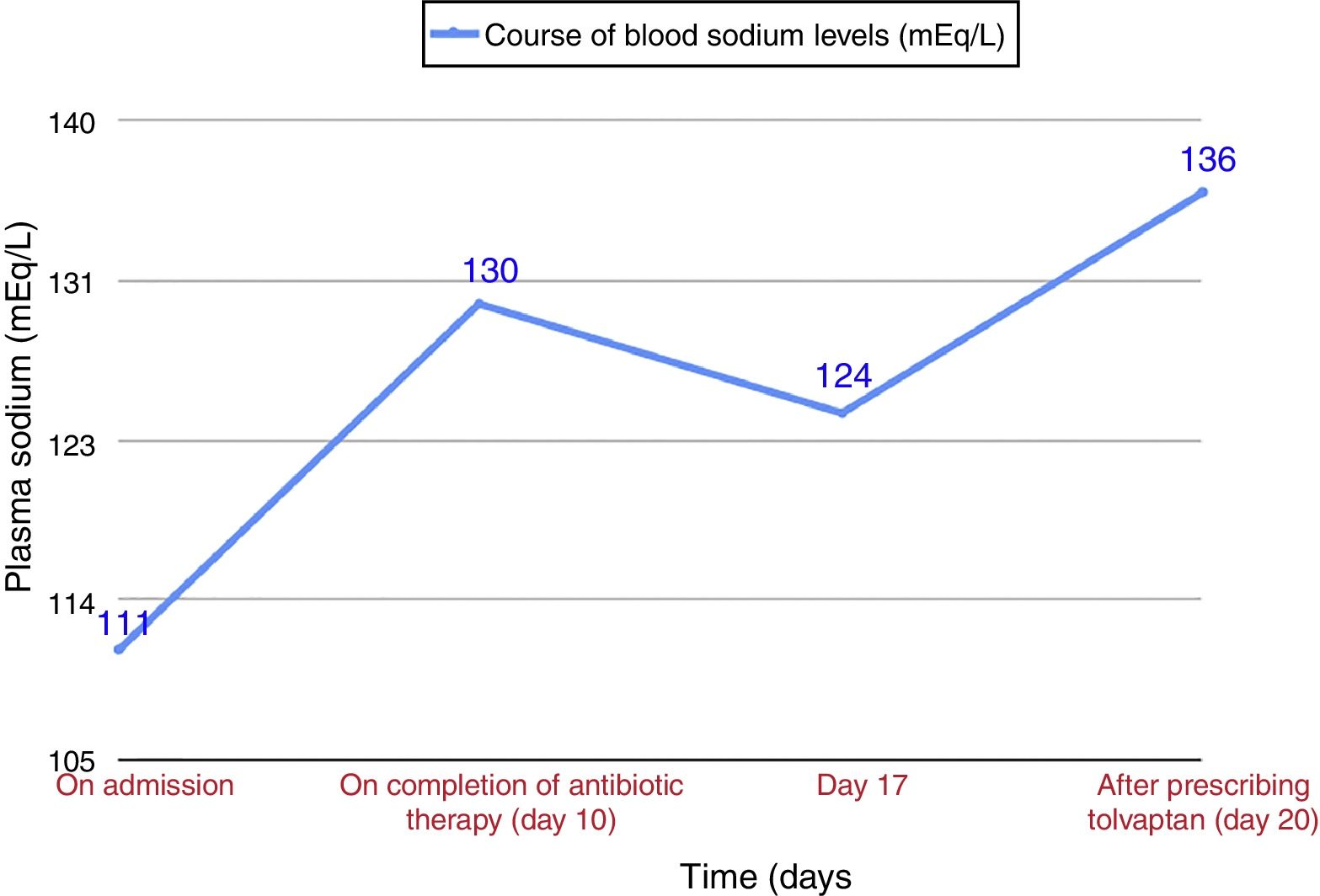
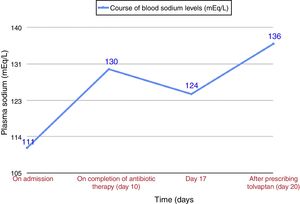
Our patient was an 84-year-old man, active smoker, with a history of benign prostate hypertrophy and exacerbator phenotype COPD with emphysema, and very severe obstruction (FEV1 27%), receiving treatment with siladosin, omeprazole, and glycopyrronium/indacaterol. He presented with intense dyspnea and cough with greenish expectoration. The only finding of note on physical examination was the presence of disperse rhonchi in both hemithoraxes and the absence of edema or signs of fluid overload. O2 saturation was 89% with home oxygen therapy at 2l per minute (lpm). Clinical laboratory tests showed microcytic anemia (hemoglobin 10g/dl), plasma sodium 111mEq/l (normal value [NV]: 135–155mEq/l), plasma osmolarity 229mOsm/l (NV: 280–300), PCR 64mg/l (NV: 0–5), normal creatinine levels, urinary sodium 76mEq/l (NV: 54–150), and urine osmolality 273mOsml/l (NV: 30–1400); arterial blood gases with O2 at 2lpm: pH 7.35, pCO2 54.8mmHg, pO2 57mmHg, and HCO3 29.7. Chest X-ray showed clamping of both costophrenic angles, signs of air trapping, and a slight increase in density reflecting scarring in the right lower lobe, present in previous X-rays. Given the suspected respiratory infection and the patient's history of COPD with very severe obstruction, treatment began with oxygen therapy, aerosol therapy, and beta-lactam and macrolide antibiotics. For the hyponatremia, water restriction at less than 500cc/day was prescribed (according the Furst formula6), plus diet with salt, furosemide, and hypertonic saline. The patient's sodium levels in blood did not normalize after completion of COPD treatment (Fig. 1), and no changes were observed on physical examination. Repeat clinical laboratory tests showed plasma sodium 124mEq/l, plasma osmolarity 247mOsm/l, urine sodium 62mEq/l, and urinary osmolarity 372mOsm/l, with normal thyroid and adrenal function. After ruling out other possible causes, a diagnosis of SIADH due to an exacerbation of emphysema phenotype COPD was given, and treatment began with tolvaptan 15mg/24h, leading to normalization of blood sodium in 4 days. The dose was reduced to 7.5mg/24h, and sodium levels in blood were normal in subsequent visits.
SIADH is characterized by the sustained release of arginine-vasopressin (ADH) in the absence of the usual stimuli, particularly hyperosmolarity and hypovolemia. Diagnosis is based on hyponatremia, plasma hypoosmolarity, urinary sodium >40mmol/l, and urinary osmolarity >100mOsm/kg, after ruling out processes that involve loss of effective blood volume (heart failure, cirrhosis with ascites, etc.), and normal renal, adrenal, and thyroid function has been confirmed.6
Symptoms are non-specific, and can range from nausea, dizziness, general malaise, agitation and confusion to seizure or coma in cases of sudden onset or very low blood sodium levels.6 SIADH is the most common cause of hyponatremia, and its heterogeneous etiology7 includes most importantly infections, drugs, tumors8 (particularly small cell lung carcinoma), COPD, and asthma. Hyponatremia due to SIADH occurs quite frequently in small cell carcinoma, sometimes as a first manifestation,9–11 and is associated with decreased survival.12
Both COPD and small cell lung carcinoma are a cause of SIADH and strongly associated with smoking, so blood sodium must also be monitored in patients with COPD and a heavy accumulated consumption of tobacco.
Treatment of hyponatremia due to SIADH differs, depending on two different clinical scenarios. In acute situations with moderate/severe symptoms (sleepiness, confusion, stupor, respiratory distress), and plasma Na+ <120mEq/l, treatment should begin with 3% hypertonic saline.13 The initial infusion rate will be 0.5mg/kg/h or 1–2ml/kg/h, depending on neurological signs.6 Treatment with tolvaptan (a selective vasopressin V2-receptor antagonist) may be considered, depending on progress. When SIADH develops with mild hyponatremia, water restriction and furosemide should be considered, and in patients who are not candidates for these measures or whose clinical symptoms persists, the use of tolvaptan is recommended.14
COPD exacerbation is a cause of SIADH, as demonstrated by Chanela et al.3 However, in our patient, hyponatremia persisted for almost 10 days after resolution of the infectious COPD exacerbation, and in the follow-up visit the patient continued to require tolvaptan to maintain normal sodium levels. A more typical course in SIADH due to COPD exacerbation would have been transient hyponatremia that normalized after resolution of the exacerbation.
We believe that this case of SIADH associated with a COPD exacerbation illustrates the need to include blood sodium monitoring in the management of these patients, in order to detect and reduce the morbidity and mortality of this fluid-electrolyte imbalance.15 It is also important to remember that this syndrome can, albeit rarely, be the first manifestation of lung cancer.
Please cite this article as: Martín Guerra JM, Martín Asenjo M, Sánchez Muñoz LÁ, Dueñas Gutiérrez CJ. La hiponatremia en la EPOC, una complicación poco conocida. Arch Bronconeumol. 2018;54:391–393.