Hypersensitivity pneumonitis (HP) or extrinsic allergic alveolitis is a pulmonary interstitial disease mainly caused by sensitization to a variety of inhaled organic particles.1 The airborne antigens which most commonly lead to the development of this hypersensitivity reaction are thermophiles, molds, and avian antigens.1 However, some cases caused by non-inhaled medications have also been reported, including exposure to bacillus Calmette-Guérin (BCG) in the treatment of urothelial bladder carcinoma,2 such as the one we describe here.
A 73-year-old man, former smoker (accumulated consumption of 30 pack-years), arterial hypertension, with no known drug allergies. He did not report any occupational or environmental exposure to birds, feathers or other organic substances. He had been diagnosed 3 months previously with superficial papillary urothelial carcinoma and was receiving treatment with intravesical BCG. He was admitted with a 10-day history of acute clinical symptoms, consisting of general malaise, deterioration, and fever 39°C, coinciding with the eighth instillation of BCG. Clinical laboratory results showed leukocytes 11900 (neutrophilos 81%), C-reactive protein 87mg/dl, and elevated liver function markers (GGT and AP). Tumor markers and angiotensin converting enzyme were normal. Cultures of sputum, urine, bronchoalveolar lavage (BAL), and blood, including Löwenstein-Jensen medium, were negative, as were pneumococcal and Legionella urinary antigen testing. Immunoglobulins (Ig) G and M were normal. Serum IgG (precipitins) for molds, birds, and feathers were negative. Chest HRCT revealed a ground glass pattern in both upper lobes, small centrilobular nodules, and consolidations in the lung bases (Fig. 1(A)). Cell distribution in BAL was: alveolar macrophages 44% and lymphocytes 56%. Flow cytometry immunophenotyping of the lymphocyte population showed: CD3+ 97.58%; CD4+ 91.88%; CD8+ 5.39%; and CD4/CD8 ratio 17. Mucosa and bronchial cartilage were retrieved by transbronchial biopsy. Spirometry was normal. Serum levels of specific anti-BCG IgG were detected using 2 different immunological techniques, double immunodiffusion (DID), and Western blot.
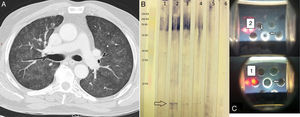
(A) Chest HRCT. Pulmonary parenchymal window shows a ground glass pattern and small centrilobular nodules in both lung fields. (B) Western blot. Lane 1 shows the serum of an asymptomatic individual receiving BCG; lanes 2, 3, and 4 show the index case after 3, 7, and 51 of corticosteroid therapy. Lane 5 shows the serum of an individual with a history of tuberculosis, and lane 6 is that of a healthy control. The arrow indicates a single band (specific anti-BCG antibodies). (C) Double immunodiffusion of sera 1 and 2. Arrows indicate precipitation bands.
Treatment began with methylprednisolone 40mg/24h, with improvement of pulmonary lesions, and the BCG instillations were discontinued. The clinical picture did not reappear after withdrawal of the corticosteroid treatment.
BCG is an attenuated live strain of Mycobacterium bovis used in the intravesical treatment of superficial bladder cancer.3 It is well tolerated and effective, but not completely free of side effects. HP as a rare complication of this immunotherapy has been detected in 0.7% of cases.4
For diagnosis, certain clinical, radiological, histological, and immunological criteria are needed. Clinical criteria tend to be non-specific. Chest HRCT shows a ground glass pattern, centrilobular nodules, and areas of consolidation. Lung biopsy obtained by bronchoscopy generally reveals non-caseifying granulomas, and BAL often contains >40% lymphocytes. It is very important to ensure that the patient has not developed miliary pneumonitis due to BCG, so presence of the bacillus must be ruled out using microbiological techniques.
The presence of specific antibodies against BCG is one of the major diagnostic criteria.1–5 For this reason, we performed a specific anti-BCG antibody assay using DID and Western blot (Fig. 1B and C), which detected a specific band in our patient but not in the controls (a symptom-free bladder cancer patient receiving BCG and a tuberculosis patient). After the patient received steroid treatment, the intensity of the specific bands could be seen to diminish on the DID, and to a lesser extent on the Western blot, indicating a reduction in antibody concentrations.
In our case, then, in the absence of a diagnostic transbronchial biopsy, evidence of high-intensity lymphocytic alveolitis, suggestive clinical symptoms, and the presence of specific anti-BCG antibodies were key to reaching a diagnosis.
A review of PubMed between 1966 and 2013 reveals that this is first time 2 different techniques, along with a control patient receiving the same treatment, have been used to demonstrate the presence of anti-BCG antibodies possibly causative of HP.
As in any HP caused by a known agent, the main treatment is withdrawal of the causative agent and administration of oral corticosteroids.
Please cite this article as: Carrasco Hernández L, Castaño Núñez ÁL, Rodríguez Portal JA. Neumonitis por hipersensibilidad como complicación del tratamiento con BCG intravesical por carcinoma de vejiga. Arch Bronconeumol. 2016;52:445–446.