Pulmonary veno-occlusive disease (PVOD) is a rare form of pulmonary arterial hypertension. The incidence of this entity is unknown, partly due to underdiagnosis and mistaken classification as idiopathic pulmonary arterial hypertension (IPAH).
PVOD is distinguished by a marked reduction in carbon monoxide diffusing capacity (DLCO) and a typical radiological pattern. It occurs more often in men, and has a more aggressive course than IPAH.1 Multiple causes, including genetic alterations, have been associated with its development. Recently, homozygous or compound heterozygous mutation of the EIF2AK4 gene was described as the cause of PVOD. This mutation appears to occur in 25% of sporadic cases and 100% of familial cases, showing an autosomal recessive inheritance pattern and high penetrance.1
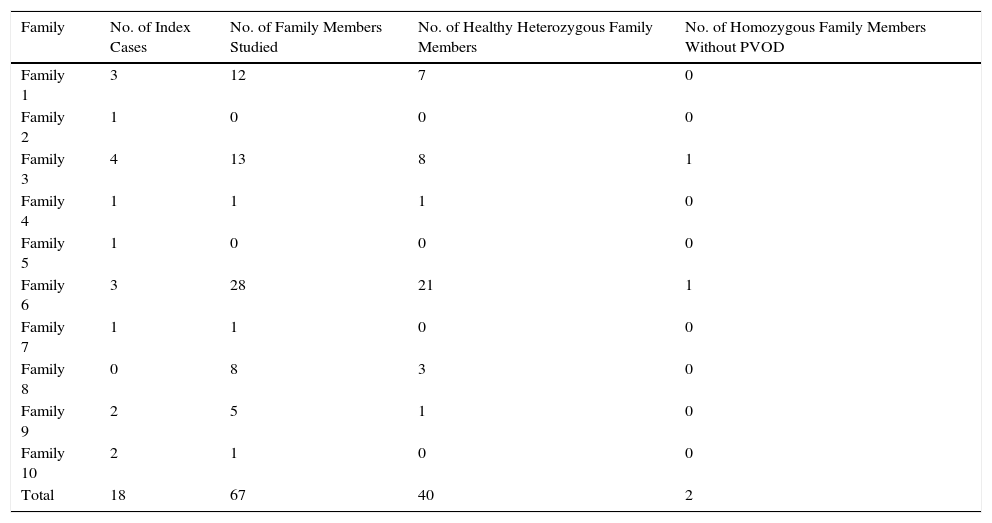
Our group has described a homozygous founder mutation C.3344C>T(p. Pro1115Leu) in EIF2AK4 in 18 patients from 10 highly consanguineous Romani families with several affected members (Table 1).2
Summary of Families Studied.
| Family | No. of Index Cases | No. of Family Members Studied | No. of Healthy Heterozygous Family Members | No. of Homozygous Family Members Without PVOD |
|---|---|---|---|---|
| Family 1 | 3 | 12 | 7 | 0 |
| Family 2 | 1 | 0 | 0 | 0 |
| Family 3 | 4 | 13 | 8 | 1 |
| Family 4 | 1 | 1 | 1 | 0 |
| Family 5 | 1 | 0 | 0 | 0 |
| Family 6 | 3 | 28 | 21 | 1 |
| Family 7 | 1 | 1 | 0 | 0 |
| Family 8 | 0 | 8 | 3 | 0 |
| Family 9 | 2 | 5 | 1 | 0 |
| Family 10 | 2 | 1 | 0 | 0 |
| Total | 18 | 67 | 40 | 2 |
PVOD: pulmonary veno-occlusive disease.
All patients developed the disease as young adults (mean: 27.43±7.3 years), and most progressed rapidly to a fatal outcome (death or double-lung transplantation) in the first year after diagnosis.
Although the clinical characteristics of the patients varied on diagnosis, they all had a common trait: severely reduced DLCO.
It is interesting to note that the study of family members revealed a high incidence of death among relatives with no genetic studies but with a history suggestive of PVOD. Moreover, we found an alarming number of family members (59.7%) who were heterozygous carriers of the mutation, generating a risk of new homozygous cases in future generations (Table 1).
At the current time, the Romani population in Spain, a community characterized by a high level of consanguinity, is estimated to be around 750000 individuals distributed around the whole country.3,4 Since this EIF2AK4 mutation appears to be typical of the Romani race, and in view of the severity of the disease, we are facing a potentially serious public health problem among this population, which could be partially prevented by early genetic diagnosis and appropriate genetic counseling aimed at reducing the number of new cases.
Therefore, we believe that maintaining a high level of suspicion is essential for Spanish physicians: and that PVOD must be ruled out and a genetic study for EIF2AK4 should be performed (as lung biopsy is contraindicated) in those Romani patients presenting with dyspnea and a family history of PAH or severely diminished DLCO. If EIF2KA4 homozygous mutations are found, the patient must be rapidly referred to a hospital with an available lung transplantation program being the initiation of pulmonary vasodilators contraindicated due to the high risk of triggering severe pulmonary edema. Moreover, family members of carriers of this mutation must be screened and given appropriate genetic counseling, in order to avoid new cases in future generations and to prevent the propagation of this devastating disease.
FundingCardiovascular Research Network (RIC) of the Instituto de Salud Carlos III, the Spanish Pulmonary Hypertension Association, Actelion and the Fundación Air Liquide.
Please cite this article as: Navas P, Rodriguez Reguero JJ, Escribano Subías P. Hallazgo de la mutación fundadora C.3344C>t(p.Pro1115Leu) en el gen EIF2KA4 en pacientes ibéricos de etnia gitana con enfermedad veno-oclusiva pulmonar: una llamada de atención a nuestra práctica diaria. Arch Bronconeumol. 2016;52:444–445.