To the Editor:
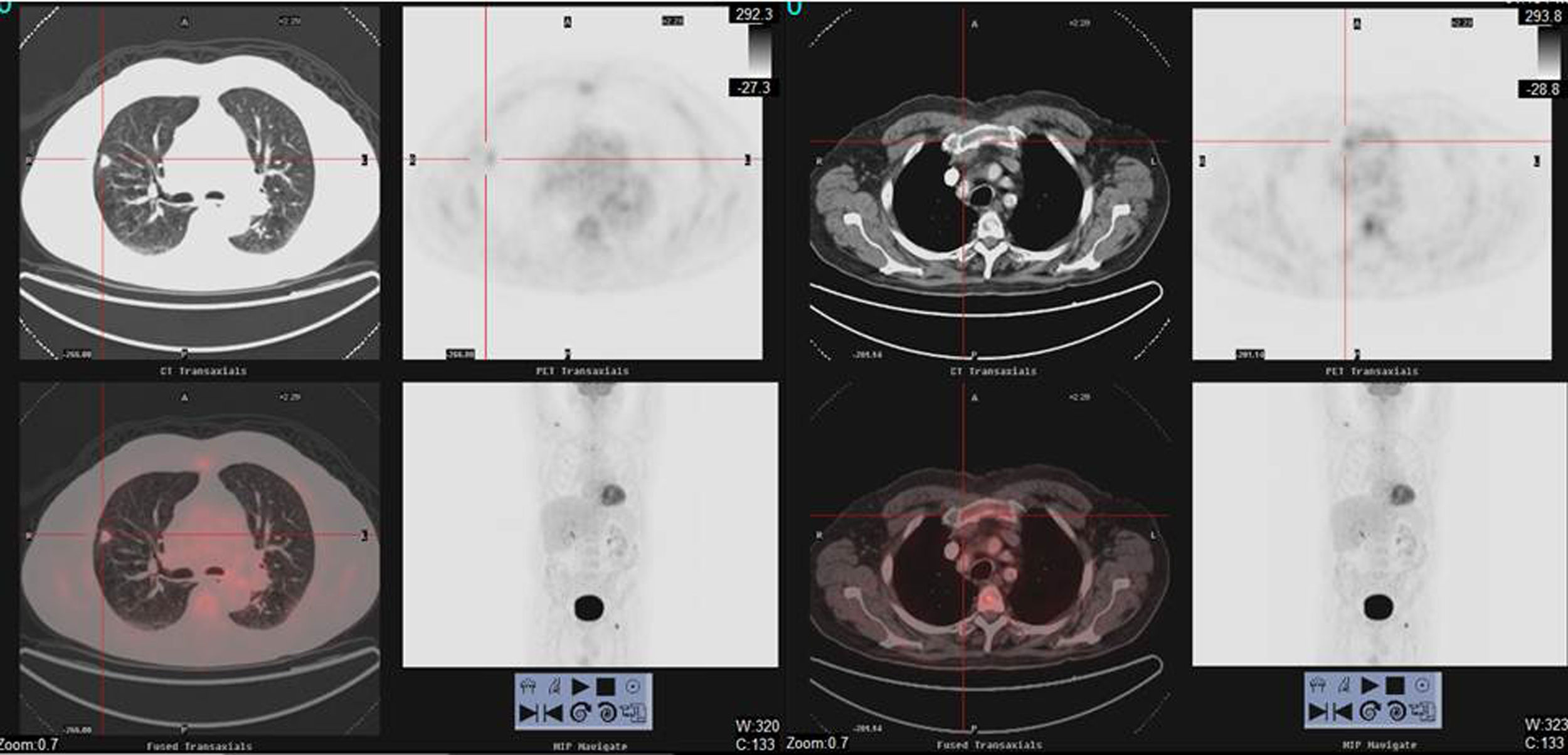
We report the case of 77-year-old man, former smoker with a cumulative index of 50 pack-years, history of dyslipidemia, IgG lambda monoclonal gammopathy of uncertain significance (MGUS) (monoclonal component [MC]: 4.8g/l) diagnosed in 2014, stable, with no evidence of progression, monitored annually by hematology, and moderate COPD, non-frequent exacerbator phenotype, receiving bronchodilator therapy, with mMRC dyspnea grade 1, and no previous admission due to exacerbation, and no respiratory symptoms or accompanying constitutional syndrome. In November 2017, after a routine chest X-ray revealed a solitary pulmonary nodule, a computed tomography (CT) scan of the chest was performed that showed a spiculated nodule measuring 13mm in the anterior segment of right upper lobe (Fig. 1), in contact with the pleura, and with retraction of the adjacent horizontal fissure. Laboratory blood tests (biochemistry, including total proteins, immunoglobulins, serum proteins, free light chain index, complete blood count, and coagulation) were normal. A positron emission tomography (18F-FDG-PET/CT) showed a solid hypermetabolic nodule (spiculated SUVmax 7.5) measuring 14mm in punctiform contact with the pleura and fissure, suggestive of primary neoplastic etiology (Fig. 1). Multiple hypermetabolic bone lesions of lytic component were observed at the distal end of the right clavicle (SUVmax 8.4) (Fig. 1) and in the D3 vertebral body, and lesions with a blastic appearance were seen in the left femoral head/neck union (SUVmax 12). These lesions were initially thought to have a metastatic origin. Moreover, an irregular tracer distribution was described in other bony structures that could not be attributed to morphological lesions, and discrete metabolic activity was detected in the sternal manubrium, so a secondary etiology could not be ruled out. A CT-guided transthoracic biopsy of the lung lesion was then performed, which was positive for adenocarcinoma of pulmonary origin. To complete staging (T1BN0Mx), we decided to obtain a biopsy of the lytic lesion in the right clavicle. This was positive for plasma cell cancer, consistent with plasmacytoma/multiple myeloma (MM).
18F-FDG PET/CT scan images showing (on the left) a solid spiculated hypermetabolic nodule (SUVmax7.5), in punctiform contact with the pleura and the fissure, measuring about 14mm, located in the anterior segment of the right upper lobe. Hypermetabolic bone lesion (on the right) with lytic component at the distal end of the right clavicle (SUVmax 8.4).
After receiving the results of the bone biopsy, the disease was reevaluated, showing stable MC (6.6g/l), with no other laboratory abnormalities. A 24h urine study was negative, and bone marrow aspirate showed 15 % infiltration of plasma cells with atypical morphology (anisocytosis with predominance of large, multinucleated cells, occasionally with visible nucleoli), irregularly distributed, with areas of abnormal phenotype of up to 25 %. The study was completed with a whole-body magnetic resonance imaging (MRI) scan that showed focal lesions in the T3 vertebral body and in the distal end of the right clavicle, highly suggestive of an infiltrative process, with a suspicion of diffuse spinal medullary infiltration in the T4-L4 segment.
The patient was diagnosed with concomitant lung adenocarcinoma T1BN0M0 (TNM 7th edition) and MGUS transformed to symptomatic IgG lambda-type oligosecretory multiple myeloma with bone involvement, ISS stage 1.
The patient had IgG lambda MGUS, an entity that occurs at a prevalence of 1–3 %. It is defined as the incidental finding of a monoclonal component in serum in a patient without diagnostic criteria for multiple myeloma, Waldenström macroglobulinemia, amyloidosis, or other lymphoproliferative disorders, and must meet the following diagnostic criteria: serum MC<30g/l, plasma cells in bone marrow <10 %, and absence of lesions in target organs (CRAB criteria: hypercalcemia, renal failure, anemia, bone lesions, amyloidosis). The risk of progression to multiple myeloma or amyloidosis is 1 % per year, with a real probability of malignant transformation at 20 years of 11 %. Factors associated with a greater risk of progression are the level of MC, IgA type, altered free light chain index (kappa/lambda), progressive type, and presence of aberrant phenotype in >90 % of plasma cells in the bone marrow.1,2
Multiple myeloma is characterized by >10 % bone marrow infiltration by plasma cells that secrete MC in serum and/or urine, along with cytokines that can cause bone lesions. Incidence is 3–5 cases/100,000 inhabitants/year, and the average age of patients is 65 years. Symptomatic multiple myeloma is defined as the presence of MC in serum and/or urine, plasma cell infiltration of the bone marrow or plasmacytoma and evidence of organ damage (CRAB), while asymptomatic multiple myeloma is defined as the presence of MC in serum and/or urine and plasma cell infiltration in bone marrow/plasmacytoma with no organ damage (CRAB).1–4
The patient received 5 sessions of stereotactic radiation therapy to the lung lesion (total dose: 55Gy). He remained disease-free at 15 months, and subsequently initiated treatment with curative intent with lenalidomide and dexamethasone, achieving complete MM remission after 12 cycles of treatment.
In conclusion, patients with a history of MGUS who present bone lesions (although initially attributed to metastatic origin) and who remain asymptomatic should undergo guided bone biopsy, whenever possible, to rule out metastatic disease and/or progression to MM, since the therapeutic approach and the prognosis of the disease vary greatly, ranging from proposed palliative treatments to curative attempt. In these cases, multidisciplinary study and follow-up of patients is essential in order to optimize the diagnostic and therapeutic procedures.
Please cite this article as: Martín-Ontiyuelo C, Sánchez-Font A, Gimeno E, Suárez-Piñera M, Curull V. Hipercaptación ósea en 18F-FDG-PET/TC en paciente con cáncer de pulmón, ¿es siempre una metástasis? Arch Bronconeumol. 2020;56:51–52.