IgG4-related disease (IgG4-RD) is an uncommon systemic disorder characterized by sclerosing lesions that can affect almost any anatomical site.1 Pulmonary involvement has highly variable clinical and radiological presentations.2 Although IgG4-related lung disease is usually preceded or accompanied by multi-organ involvement,3 rare cases of solely lung involving IgG4-RD have been described.4•6 Herein, we report two biopsy-proven cases of IgG4-RD with lung mass as the sole radiographic presentation.
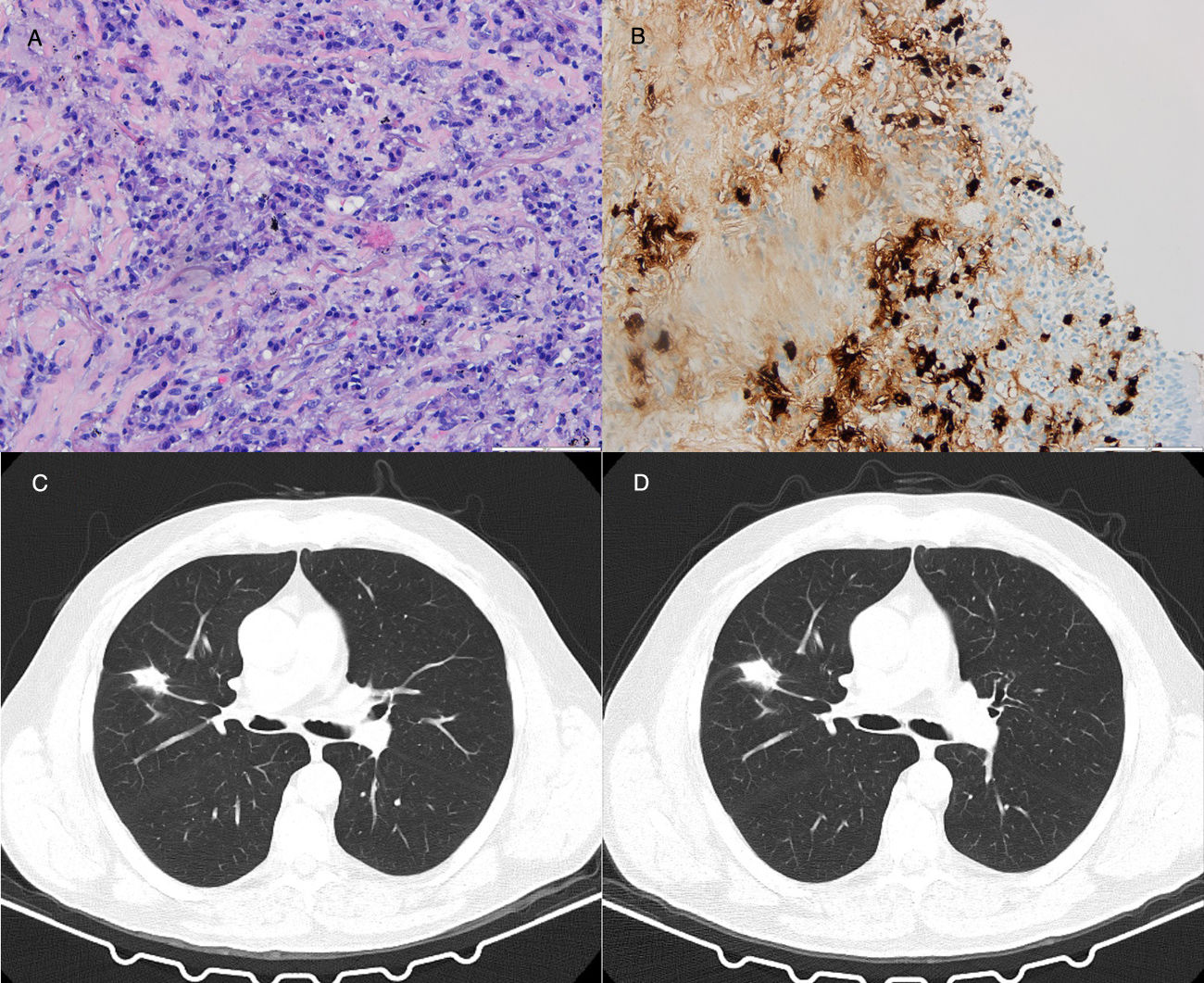
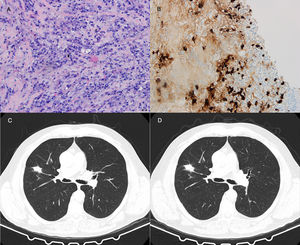
64-Year-old man with a 27mm spiculated mass in the right upper lobe on computed tomography (CT) in the setting of chronic cough with hemoptoic sputum. He was a previous smoker (100 pack-year of smoking). Bronchoscopy showed neither signs of bleeding nor morphological anomalies and bronchoalveolar lavage (BAL) was negative for malignant cells and acid-fast bacilli. Further investigation with positron emission tomography (PET) demonstrated a right upper lobe nodule with standardized uptake value (SUV) max of 2.6. Due to the high suspicion of primary lung malignancy, biopsy of the nodule was performed by transthoracic needle aspiration (TTNA), documenting a fibrocollagenous lesion with lymphoplasmacytic infiltration and wall thickening of venous-type vessels. On immunohistochemistry, the number of IgG4-positive plasma cells was >20 per high power field (HPF) and immunostaining for CD20 and CD3 was positive. The serum concentrations of total IgG and IgG4 were normal.
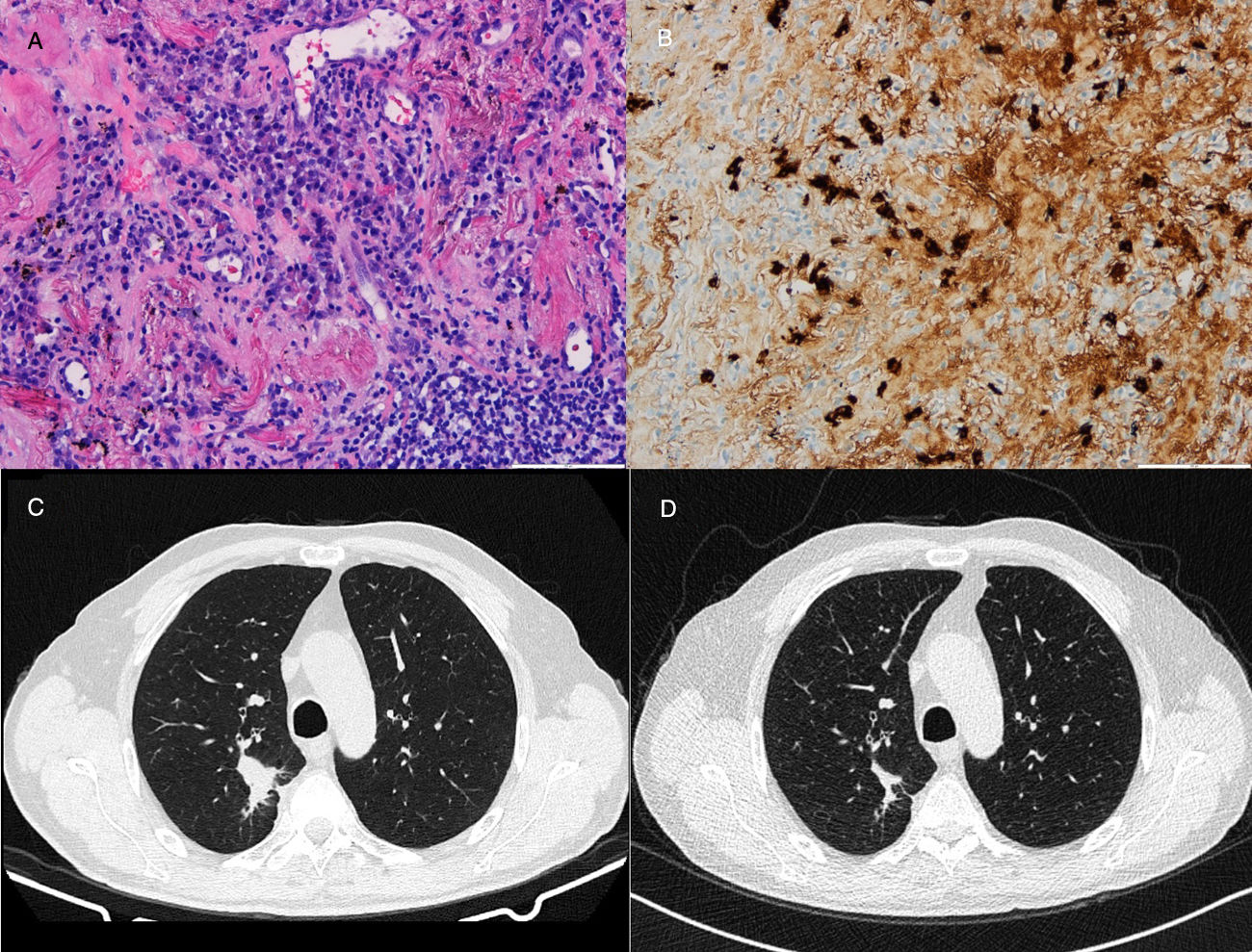
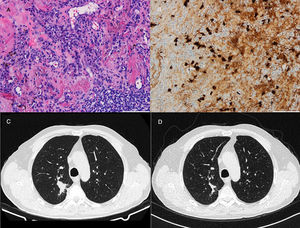
71-Year-old man who presented with posterior upper thoracic pain for 2 years. He is an active smoker with 50 pack-year of smoking. Chest CT detected a 35mm spiculated mass in the right upper lobe, which was biopsied by TTNA. Histological examination demonstrated a nodular lesion with total architectural distortion due to fibrosis and lymphoplasmacytic infiltration. On immunohistochemistry, the number of IgG4-positive plasma cells was >30 per HPF. Given the suspicion of IgG4-RD further workup was performed, showing elevated serum concentrations of IgG4 and normal concentrations of total IgG. PET demonstrated a right upper lobe nodule with SUV max of 3.1 and BAL pathological analysis was negative for malignant cells. Hence, in both cases the diagnosis of IgG4-RD was established based on clinico-pathological correlation.
Both patients started corticosteroid therapy with an initial prednisolone dosing of 0.6mg/kg/d for 4 weeks. Patient 1 had a poor compliance to the treatment and presented no improvements after 2 months (Fig. 1). Patient 2 showed clinical improvement and a reduction of the mass tm) dimensions on CT imaging after 3 months of treatment (Fig. 2). The prednisolone dose was tapered by 10mg/kg/d after the first and third month of therapy, maintaining 10mg/d at the present time.
Although the epidemiology of IgG4-related lung disease (IgG4-RLD) remains poorly described,7 it usually occurs in male adults with an average age of 69 years.8 Its clinical presentation depends on the location of the lesion, nonetheless half of patients present nonspecific respiratory symptoms, whereas the remaining present abnormalities on imaging studies in the absence of symptoms.2,8
Appropriate diagnosis can be challenging, as it relies upon the integration of clinical, laboratorial and histopathologic findings. The consensus statement on the pathology of IgG4-RD mentions that the final diagnosis requires both an appropriate histologic appearance and increased numbers of IgG4+ plasma cells.1 Such statement suggested >50/HPF and >20/HPF as the cut-off value for increased IgG4+ cells in surgical and nonsurgical biopsies, respectively.1 Additionally, it underlines that diagnosis should be based primarily on morphological appearance and less importantly on tissue IgG4+/IgG+ ratio, since various conditions can course with elevated IgG4+/IgG+ ratio.1,2 While pathologic findings represent the cornerstone for a definite diagnosis, the interpretation of lung biopsy for any fibroinflammatory condition is challenging due to the fact that the lung tends to undergo stereotypic morphologic responses regardless of the type of injury.8 The characteristic histologic findings of IgG4-RLD are fairly common in lung samples afflicted by severe infection or organizing injury of various causes,8 highlighting the importance of a careful correlation with clinical and laboratorial data.
Elevation of IgG4 serum concentration is used to support the diagnosis of IgG4-RD.2 However, recent studies have demonstrated that up to half of patients with biopsy-proven and clinically active IgG4-RD may have normal serum concentrations9 and only a minority of patients with high IgG4 levels have IgG4-RD.10 Thus the current trend is to deemphasize excessive reliance on serum IgG4, which is neither specific nor sensitive of IgG4-RD.11
PET has been advocated as it can detect unforeseen localizations of the disease and assess the extent of systemic disease.6 In both patients, PET confirmed that the disease is confined to the lung. Two other case reports documented pulmonary, hilar and mediastinal lesions with SUV max from 2.1 to 11.0,6,12 yet there is no demonstrated range for SUV that can either gauge disease activity or guide treatment decisions.7
The natural course of the disease is not completely known and there are no formal treatment guidelines. However, it is agreed among experts that the threshold for initiating treatment is low, in order to prevent fibrosis and its irreversible damage on organs.7 The consensus statement on the treatment of IgG4-RD recommends glucocorticoids as the first-line agent for remission induction in all patients with active and untreated disease.7 Prednisolone at an initial dosage of 0.6mg/kg/d for 2•4 weeks is recommended,13 which may be adjusted if the disease appears to be particularly aggressive.7 Immunossupression with rituximab is indicated in the steroid refractory disease.2 There is no consensus regarding the tapering regimen and maintenance therapy however.
In cases of isolated pulmonary disease, it is imperative to ensure a regular follow-up with screening of multi-systemic involvement and malignancies. Although the association of lung cancer with IgG4-RLD remains unclear, a small number of adenocarcinoma-associated cases have been reported.14
In conclusion, IgG4-RLD is a rare condition that may be diagnosed after the unexpected result of a biopsy in the setting of suspected lung malignancy. Awareness of IgG4-RD is of utmost importance, as the pathologist must perform a specific immunostaining and the clinician must exclude other differential diagnoses. Increasing recognition and further studies will enlighten our understanding of the pathogenesis, diagnostic criteria and standardized therapy for this disease.