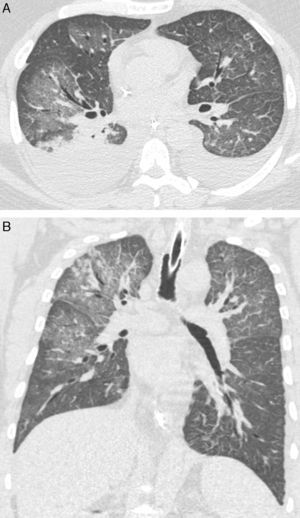
We retrospectively reviewed the records of 8 adult patients with confirmed hantavirus pulmonary syndrome (HPS), aiming at describing high-resolution computed tomography (HRCT) findings. The patients were examined between 2003 and 2014 in 6 tertiary hospitals in Brazil. Diagnoses of HPS were based on medical histories, clinical course, and imaging findings. Serological tests (ELISAs) were positive for Hantavirus in all patients. Although the HRCT findings of Old World hantaviruses have been well described, to our knowledge no study has examined HRCT findings in a series of patients with HPS. In our study, the main HRCT findings were ground glass opacities (GGOs) and smooth interlobular septal thickening, observed in all patients (Fig. 1). However, the crazy-paving pattern was observed in only 3 cases. Pleural effusion and peribronchovascular thickening were observed in 5 cases. Four patients had small nodules, and only 1 had foci of consolidation. Abnormalities were bilateral and diffuse in all patients.
HPS is an emerging zoonotic disease caused by hantavirus, a spherical and enveloped single-stranded RNA virus belonging to the family Bunyaviridae. In the Americas, natural reservoirs of the virus are rodents of the subfamily Sigmodontinae.1,2 The genus Hantavirus consists of several viruses classified into 2 groups, each associated with a different clinical syndrome: Old World hantaviruses, which cause hemorrhagic fever with renal syndrome or nephropathia epidemica; and New World hantaviruses, related to HPS.1,3 HPS was first recognized as a clinical entity in the United States in 1993. On average, approximately 200 cases of HPS per year are reported in the Americas, and although the number of cases is much smaller than that of HFRS, its average case fatality is about 40%.4
Both syndromes primarily involve young adult males, and transmission is related to occupational activities, such as veterinary medicine, farming, and related professions.1 Hantavirus can affect humans after inhalation of aerosolized virus particles from urine, saliva, or dried excreta of reservoir rodents. Person-to-person transmission has been reported in a few cases, generally associated with a specific strain, the Andes virus.5
The clinical presentation of HPS is usually nonspecific, and in most cases presents as flulike symptoms. A cough (initially nonproductive) typically signals the transition to the cardiopulmonary phase, in which a fulminant capillary leak syndrome may lead to rapidly progressive pulmonary edema and shock.5 Clinical manifestations develop after an incubation period of about 2–3 weeks, and usually comprise 3 well-demarcated phases. The prodromal phase is characterized by nonspecific, flulike manifestations that last from 2 to 5 days. The cardiopulmonary phase typically starts with a dry cough that rapidly becomes productive, with mucus and bloody sputum, along with respiratory failure and cardiovascular shock. Laboratory findings during this phase include marked leukocytosis with a leftward shift, thrombocytopenia, elevated serum lactate dehydrogenase and aspartate aminotransferase levels, hemoconcentration, and low serum albumin concentration due to capillary leakage.1–3,5 Improvements in oxygenation, diuresis, and hemodynamic stabilization are the hallmarks of the convalescent phase. Although a presumptive diagnosis of HPS can be made based on the patient's clinical history and radiologic findings, confirmation of the diagnosis requires virus-specific diagnostic tests, such as serological tests (ELISA), reverse transcription, and/or PCR. ELISA results for all patients in our sample were positive for Hantavirus.1,3
In conclusion, the predominant HRCT findings in patients with HPS were GGOs and smooth septal thickening. In the appropriate clinical setting, these findings are of great help in the diagnosis. Pleural effusion and peribronchovascular thickening are also frequent, but less characteristic findings.
Please cite this article as: de Lacerda Barbosa D, Zanetti G, Marchiori E. Síndrome pulmonar por Hantavirus: hallazgos en la tomografía axial computarizada de alta resolución. Arch Bronconeumol. 2017;53:35–36.