The treatment of choice for alveolar proteinosis in children is whole-lung lavage, by which proteinaceous material deposited in the alveoli is removed by instilling saline solution directly into the lung.1 This procedure, which is rarely performed in the pediatric setting and for which no specific equipment has been developed, is a true technical challenge.2
We report the case of a female infant who was healthy until the age of 7 months, when she was diagnosed with acute myeloblastic leukemia type M5. She received a haploidentical stem cell transplant (SCT) from her father at the age of 13 months in her first full remission. She developed a post-SCT complication of generalized host-versus-graft disease, requiring intensive immunosuppressive treatment. Two months after the SCT, a chest computed tomography (CT) performed for persistent fever was normal.
She developed respiratory disease at the age of 20 months (7 months post-SCT), following Klebsiella pneumoniae sepsis, with progressive breathing difficulties and hypoxemia.
As her respiratory symptoms failed to improve despite antibiotic therapy, a chest CT was performed that showed a bilateral “crazy-paving” appearance. The patient was anesthetized and a simultaneous bronchoalveolar lavage (cytology showed abundant, dense granular material, PAS staining positive; Pseudomonas aeruginosa was grown on culture) and lung biopsy via mini-thoractomy were performed. This allowed us to rule out infection and interstitial lung disease due to surfactant deficit, and to confirm the diagnosis of alveolar proteinosis.
Given these findings, we decided to perform therapeutic lung lavage. A total of 2700ml of warm saline solution was delivered to the right lung in 12 aliquots of 27ml/kg. Two weeks later, the procedure was repeated in the left lung, using around 2500ml.
Given the unavailability of double-lumen tubes for children under 8 years old, we decided to introduce 2 endotracheal tubes using direct laryngoscopy:
- –
One, 3.5mm in diameter, was placed in the trachea to maintain ventilation.
- –
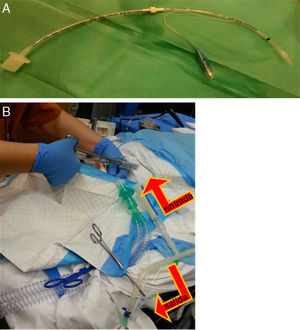
The other, 3mm in diameter, with a balloon, was placed in one of the main bronchi, and telescoped until it reached the correct length and caliber; this one was used for the instillation of serum (Fig. 1A).
Fig. 1.(A) Endotracheal tube, 3mm with balloon, extended by connecting it with another 3.5mm tube, through which the warm saline solution was instilled and collected after it was placed in the main bronchus. (B) Image showing the tube located in the trachea, used to ventilate the patient, and the tube placed in the main bronchus, used to instill the saline solution with an alternating clamping system (the outflow was clamped at the time of instillation and the inflow was clamped at the time of emptying the lung fluid), indicated by the arrows.
The correct placement of both tubes was confirmed with flexible bronchoscopy and fluoroscopy.
Fluid was introduced and removed from the bronchus by gravity; the tubes were alternately clamped to allow entry or exit of fluid (Fig. 1B).
After each procedure, the patient was admitted to the intensive care unit for 12h. She received corticosteroids to prevent laryngeal edema and was extubated after a few hours without complications.
Clinical response after lavage of both lungs was very good, and her hypoxemia resolved rapidly. Two weeks after the procedure, a high-resolution, low-radiation CT of the chest was performed, revealing persistent ground-glass lung lesions, but to a lesser extent than before.
The presence of autoantibodies against granulocyte monocyte-colony stimulating factor (GM-CSF) in blood was ruled out, and GATA2 mutation testing was also normal.
Five months after the procedure, lung lavage was repeated due to reappearance of hypoxemia, and the patient again showed clinical improvement.
We use this case to describe the procedure and equipment used for whole-lung lavage in an infant, with practical suggestions for performing the technique, including the simultaneous use of 2 endotracheal tubes, one of which was lengthened to allow selective intubation. This system was selected because partial lavage with instillation of smaller aliquots of saline solution via the bronchoscope to the different lung segments is more laborious, although this could be an option in patients with severe respiratory failure who may not tolerate whole-lung lavage.3
The trigger in our patient was thought to be a transient dysfunction of alveolar macrophages (responsible, along with type II pneumocytes, for surfactant catabolism) due to immunosuppression, causing occupation of the alveolar space.4 Another factor associated with this disease in the literature is infection, and in our case, the microorganisms in blood and in bronchoalveolar lavage may have played an important role in determining the course of the patient's respiratory disease.5
We would like to thank the medical team of the pediatric hematology unit, particularly Dr. Laura Alonso, and all our colleagues in the pediatric respiratory medicine unit, particularly Dr. Antonio Moreno and Dr. Alba Torrent, for their help in preparing this article. We would also like to thank all the operating room staff who collaborated in the organization and successful outcome of the procedure described here.
Please cite this article as: Iglesias-Serrano I, Montferrer-Estruch N, Nuño-Sanz R. Técnica de lavado pulmonar en lactante con proteinosis alveolar. Arch Bronconeumol. 2017;53:36–37.