Muscle involvement is found in most critical patients admitted to the intensive care unit (ICU). Diaphragmatic muscle alteration, initially included in this category, has been differentiated in recent years, and a specific type of muscular dysfunction has been shown to occur in patients undergoing mechanical ventilation. We found this muscle dysfunction to appear in this subgroup of patients shortly after the start of mechanical ventilation, observing it to be mainly associated with certain control modes, and also with sepsis and/or multi-organ failure. Although the specific etiology of process is unknown, the muscle presents oxidative stress and mitochondrial changes. These cause changes in protein turnover, resulting in atrophy and impaired contractility, and leading to impaired functionality. The term ‘ventilator-induced diaphragm dysfunction’ was first coined by Vassilakopoulos et al. in 2004, and this phenomenon, along with injury cause by over-distention of the lung and barotrauma, represents a challenge in the daily life of ventilated patients.
Diaphragmatic dysfunction affects prognosis by delaying extubation, prolonging hospital stay, and impairing the quality of life of these patients in the years following hospital discharge. Ultrasound, a non-invasive technique that is readily available in most ICUs, could be used to diagnose this condition promptly, thus preventing delays in starting rehabilitation and positively influencing prognosis in these patients.
La afectación muscular del paciente crítico está presente en la mayoría de pacientes que ingresan en el servicio de medicina intensiva (SMI). La alteración, en particular, del músculo diafragmático, inicialmente englobada en esta categoría, se ha diferenciado en los últimos años y se ha demostrado la existencia de una disfunción muscular propia de los pacientes sometidos a ventilación mecánica. En este subgrupo de pacientes encontramos una disfunción muscular que aparece de manera precoz después del inicio de la ventilación mecánica y que se relaciona principalmente con el uso de modalidades control, la presencia de sepsis y/o de fracaso multiorgánico. Aunque se desconoce la etiología concreta que desencadena el proceso, el músculo presenta procesos de estrés oxidativo y alteración mitocondrial que provocan un desequilibrio en la síntesis proteica, con el resultado de atrofia y alteración de la contractilidad y, como consecuencia, una menor funcionalidad. No fue, de hecho, hasta 2004 cuando Vassilakopoulos et al. describieron el término «disfunción diafragmática asociada a ventilación mecánica», que, junto a la lesión por sobredistensión pulmonar y por barotrauma, representan un reto en el día a día de los pacientes ventilados.
La disfunción diafragmática tiene influencia en el pronóstico, retardando la extubación, aumentando la estancia hospitalaria y afectando la calidad de vida de estos pacientes en los años siguientes al alta hospitalaria. La ecografía, como técnica no invasiva y accesible en la mayoría de unidades, podría ser de utilidad en el diagnóstico precoz para iniciar, de forma avanzada, la rehabilitación e influir positivamente en el pronóstico de estos enfermos.
Mechanical ventilation (MV) has been used throughout history as a basic tool in the treatment of patients with respiratory failure, and as a means of improving prognosis. Almost 40% of mechanically ventilated patients have difficulties during MV weaning, due to multiple factors. A delay in weaning can prolong the stay in the intensive care unit (ICU) and lead to a poorer prognosis1–3 and a 12% increase in mortality compared to patients with no weaning problems.4
In the last 20 years or so, attention has focused on the study of ventilation-induced diaphragm dysfunction (VIDD)5 as one of the complications associated with MV. This term refers to diaphragm dysfunction that occurs soon after initiating MV.6 VIDD worsens prognosis and is associated with extubation failure, which in turn prolongs MV7–12 and increases the risk of mortality.13–16 At present, however, diaphragm function is not routinely monitored in many units, suggesting that this entity may be systematically underdiagnosed.17
Ventilation-induced Diaphragm DysfunctionAlthough respiratory muscle weakness could be considered part of the overall muscle involvement in a critical patient, the last 10 years have seen the emergence of the concept of VIDD.18 This term refers to diaphragm muscle dysfunction caused by the negative effect of the MV itself, that can occur along with or independently of involvement of the rest of the musculature.
Although earlier studies described how complete diaphragm inactivity induced in patients receiving controlled modality ventilation leads to a rapid, progressive loss of diaphragm function,19,20 it was not until 2004 that Vassilakopoulos first coined the term VIDD,18 defined as a progressive loss of diaphragm muscle strength that occurs soon after starting MV.8,21 The condition affects up to 65% of ventilated patients,7 and is clinically significant due to its early onset.
Pathophysiology of Ventilator-induced Diaphragm DysfunctionSeveral studies have shown that the use of controlled MV (CMV), in which the patient makes no inspiratory effort and the diaphragm is not actively contracted, can lead to contractile dysfunction and diaphragm atrophy within 24h in both laboratory animals and humans.20,22,23 Atrophied muscles lose strength and the diaphragm excursion is diminished due to the reduction in the cross-sectional area of the muscle fibers, manifesting as a loss of inspiratory capacity.7,24 Below we discuss the physiopathological principles associated with VIDD.
Diaphragmatic AtrophyCMV-induced diaphragm atrophy occurs extremely rapidly.22 Significant diaphragm atrophy can be observed in laboratory animals within the first 12–18h of starting CMV, with no sign of peripheral atrophy.25 Thus, MV-induced diaphragm atrophy is significantly greater than the atrophy caused by skeletal muscle disuse.22 Levine et al. encountered similar findings in patients receiving CMV for 18–69h, and showed a significant reduction of around 53%–57% compared to healthy volunteers in both type 1 and type 2 fibers in cross-sectional diaphragm biopsies.23
Changes in Muscle Fiber UltrastructureCMV causes time-dependent changes in the ultrastructure of the diaphragm muscle fibers.19,26,27 First, areas containing abnormal myofibrils due to myofibrillar disorganization and Z-band changes appear.19 This is followed by the appearance of areas of muscle fiber regeneration, with no signs of inflammation.27 Finally, if MV is prolonged (over 3 days), cytoplasmic lipid vacuoles increase, probably due to an autophagic process.27–29
Contractile DysfunctionIn 1994, Le Bourdelles et al. were the first to describe, in an animal model, the appearance of contractile dysfunction 48h after starting CMV.30 Prolonged MV causes progressive, time-dependent loss of diaphragm strength.20 As with atrophy, this reduction in diaphragm strength can be seen within 12h of CMV.31,32 Several studies have shown that the maximum inspiratory pressure peak is lower in patients receiving prolonged MV compared to controls.18
Changes in Protein SynthesisDiaphragmatic atrophy and dysfunction associated with the use of CMV occur primarily due to a reduction in protein synthesis and an increase in proteolysis.23,33 Diaphragmatic protein synthesis can fall by 30% after only 6h of MV.33 Increased proteolysis is associated with the activation of protease pathways (calpain, capase-3, and ubiquitin-proteasome system), apoptosis pathways, and activation of autophagy.34,35 Hooijman et al. analyzed the activity of the ubiquitin-proteasome pathway in diaphragm biopsy samples from patients ventilated during thoracic surgery. Patients with a significant increase in this pathway showed a loss of approximately 25% of both slow and fast twitch diaphragm muscle fibers on cross-sectional biopsy, and reduced contractile strength.36,37
Increased Oxidative StressDuring CMV, changes occur in mitochondrial morphology causing altered functioning and excessive creation of reactive oxygen species (ROS). Increased oxidative stress triggers the activation of calpain and caspase-3 pathways in the diaphragm.34,38,39 The very early appearance (at 18 h) of reduced diaphragm muscle fiber diameter and evidence of oxidative stress phenomena and activation of proteases has been confirmed in brain-dead donor patients.23 Prolonged MV also leads to diminished antioxidant capacity in the diaphragm, reflected by reduced glutathione levels, CuZn superoxide dismutase, and peroxidase glutathione activity.40
Effect of Critical Disease on Diaphragm FunctionAlthough the consequences of CMV-induced diaphragm dysfunction are known, the pathways leading to these changes have yet to be described. Diaphragm inactivity induces progressive, time-dependent diaphragm atrophy.6,25 However, other factors also play a part in increasing the risk of VIDD and greater diaphragmatic involvement. Sepsis and multiorgan failure are the factors most robustly associated with diaphragm dysfunction, which is more severe in septic patients than in patients with shock due to other causes.7 The proinflammatory situation that occurs in these diseases may reduce protein synthesis and increase proteolysis, which itself may trigger an acute loss of muscle mass.9,41
Concomitant factors and pharmacological treatment (corticosteroids or muscle relaxants, for example) may exacerbate VIDD. Two studies examining muscle relaxants in mice confirmed that the use of rocuronium exacerbates MV-induced contractile dysfunction.42,43 Data on the effect of corticosteroids on diaphragm function are contradictory. Sassoon et al. found an association between the administration of high-dose methylprednisolone and reduced inspiratory force in an experimental model.44 However, the group led by Maes showed that administration of a single dose of corticosteroids reduced the deleterious effect of MV.42 Experimental data have associated high blood glucose levels with loss of diaphragm strength, related with an increase in oxidative stress processes.45
Invasive Mechanical Ventilation Modalities and Ventilation-induced Diaphragm DysfunctionThe diaphragmatic changes characteristic of VIDD caused by controlled modality ventilation have been demonstrated in animal models and humans, and onset is early.8,23,2633,46,47 This modality, in which the diaphragm remains totally inactive, can cause greater atrophy and contractile dysfunction, leading to loss of diaphragm strength. Unlike CMV modalities, uncontrolled MV requires the patient to make the inspiratory effort. Several authors have suggested that this action may prevent the deleterious effects of CMV. Doering et al. showed that the prolonged use of high levels of pressure in support pressure modalities caused atrophy and contractile dysfunction.48 In contrast, Futier et al. suggested that assisted MV and pressure support would reduce proteolysis and increase protein synthesis in the diaphragm.49 Gayan-Ramírez et al. demonstrated that short periods of spontaneous breathing during CMV delays the effects of this modality on the diaphragm, adding credence to the idea that diaphragm disuse is the main causative factor of VIDD.50 Changes in the configuration of the diaphragm may be prevented by maintaining inspiratory effort levels provided by pressure support.51
Non-invasive Mechanical Ventilation and High-flow Oxygen TherapyOnly 1 study has evaluated diaphragm functionality after starting non-invasive MV (NIMV). The authors conclude that the force generated by the diaphragm falls after initiating ventilation, and that this is related with high levels of pressure support.52 NIMV applies positive pressure, with a certain degree of flattening and muscle stretching, which may be the origin of the dysfunction.
High-flow oxygen therapy provides gas at up to 60l/min with the corresponding FIO2, generating low levels of positive pressure and ventilatory pattern changes, with increased circulating volume,53 but to date, no studies have analyzed its impact on diaphragm function. However, as this respiratory support system ensures the patient's diaphragm is fully activated at all times, it probably has little impact on muscle strength.
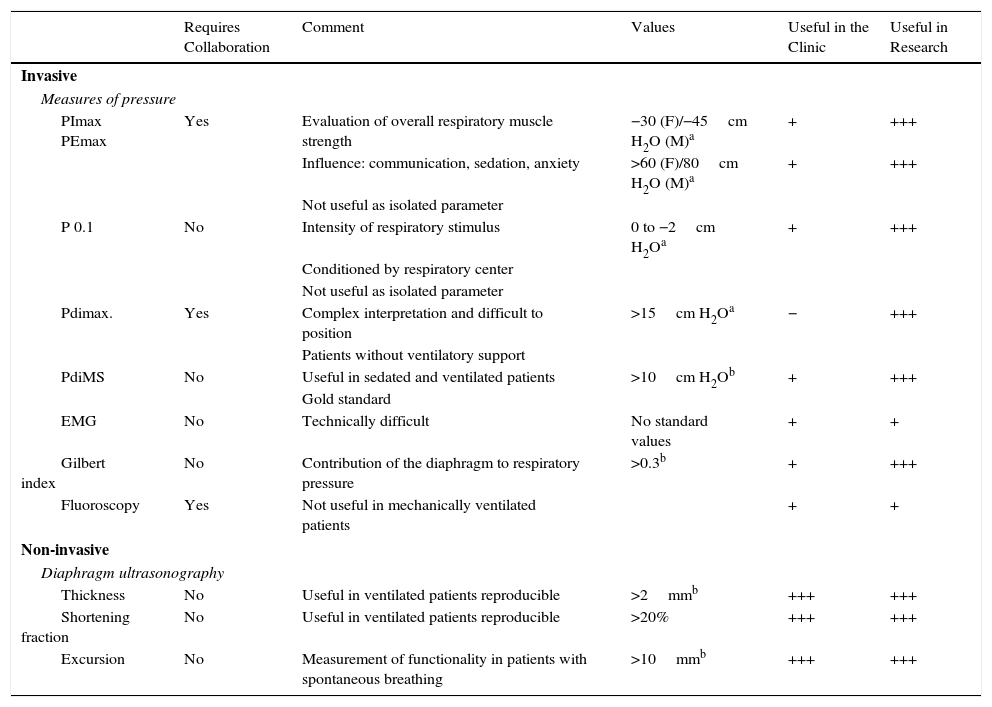
Diagnosis and MonitoringVery few UCIs conduct routine monitoring of respiratory muscle function, either because its importance is underestimated or because the technical difficulties are considerable.17 VIDD diagnosis is initially based on suspicion in mechanically ventilated patients whose intercurrent difficulties have been solved but who present problems on MV weaning. In the case of VIDD, no relationship has been established between clinical examination and the degree of diaphragm dysfunction,54 so additional examinations are needed for diagnosis (Table 1). Below is a list of some of the best tests for evaluating diaphragm function. Some are only useful in research; others can be used in clinical practice.
Tests for the Evaluation of Diaphragm Function in Mechanically Ventilated Patients.
| Requires Collaboration | Comment | Values | Useful in the Clinic | Useful in Research | |
|---|---|---|---|---|---|
| Invasive | |||||
| Measures of pressure | |||||
| PImax PEmax | Yes | Evaluation of overall respiratory muscle strength | −30 (F)/−45cm H2O (M)a | + | +++ |
| Influence: communication, sedation, anxiety | >60 (F)/80cm H2O (M)a | + | +++ | ||
| Not useful as isolated parameter | |||||
| P 0.1 | No | Intensity of respiratory stimulus | 0 to −2cm H2Oa | + | +++ |
| Conditioned by respiratory center | |||||
| Not useful as isolated parameter | |||||
| Pdimax. | Yes | Complex interpretation and difficult to position | >15cm H2Oa | − | +++ |
| Patients without ventilatory support | |||||
| PdiMS | No | Useful in sedated and ventilated patients | >10cm H2Ob | + | +++ |
| Gold standard | |||||
| EMG | No | Technically difficult | No standard values | + | + |
| Gilbert index | No | Contribution of the diaphragm to respiratory pressure | >0.3b | + | +++ |
| Fluoroscopy | Yes | Not useful in mechanically ventilated patients | + | + | |
| Non-invasive | |||||
| Diaphragm ultrasonography | |||||
| Thickness | No | Useful in ventilated patients reproducible | >2mmb | +++ | +++ |
| Shortening fraction | No | Useful in ventilated patients reproducible | >20% | +++ | +++ |
| Excursion | No | Measurement of functionality in patients with spontaneous breathing | >10mmb | +++ | +++ |
PdiMS: transdiaphragmatic pressure by magnetic stimulation of phrenic nerve; Pdimax: maximum transdiaphragmatic pressure; PEmax: maximum expiratory pressure; PImax: maximum inspiratory pressure.
Esophageal pressure is a good indicator of pleural pressure55,56 and is used together with gastric pressure to calculate transdiaphragmatic pressure (Pdi). Pdi is simply the difference between pleural pressure and abdominal pressure, and is the basis for calculating diaphragm strength.7,13,57,58 The gold standard in patients who cannot collaborate in the test is to calculate Pdi via cervical magnetic stimulation of the phrenic nerve.8,54,59 However, this is an invasive technique that is not available in most ICUs.
A reduction in Pdi can be detected soon after starting MV,59 and continues logarithmically as the duration of MV increases.8 Demoule et al. published a study7 that showed how Pdi values lower than 11cm H2O on the first day of ICU admission predicted a poor prognosis, high mortality (49%), and longer MV.7,14
Gilbert's index, calculated using Pdi (ΔGastric pressure/ΔPdi) during a normal inhalation, evaluates the contribution of the diaphragm to respiratory pressure during quiet breathing.60 Watson et al., using diaphragm ultrasonography and Pdi in patients undergoing cardiothoracic surgery, found a good correlation between low diaphragm excursion and low Gilbert indices.61
P0.1P0.1 measures the negative pressure generated by the patient against an artificial airway in the first 100ms of an inspiratory effort, reflecting the neuromuscular impulse transmitted by the phrenic nerve.62 Though not without limitations, it is a measurement of central stimulus and an indicator of diaphragm function.63,64 This measurement has been included in artificial respirators, and, as such, it has become a clinical variable accessible at the bedside.65
UltrasonographyThe need for early diagnosis with an easily available, non-invasive technique explains the growing use of ultrasonography in the evaluation and follow-up of diaphragm function and inspiratory effort.66 Its limitations include inter- and intra-observer variability and the fact that the dynamic study (diaphragm excursion) can only be conducted in patients without MV.67–70 However, several studies using ultrasonography have shown good accuracy and reproducibility for evaluating diaphragm function, both in healthy volunteers and in critical patients.52,69
Diaphragm thickness (as a sign of atrophy), shortening fraction, and diaphragm mobility studies (as a sign of diaphragm activity) are the parameters most commonly evaluated by ultrasonography.
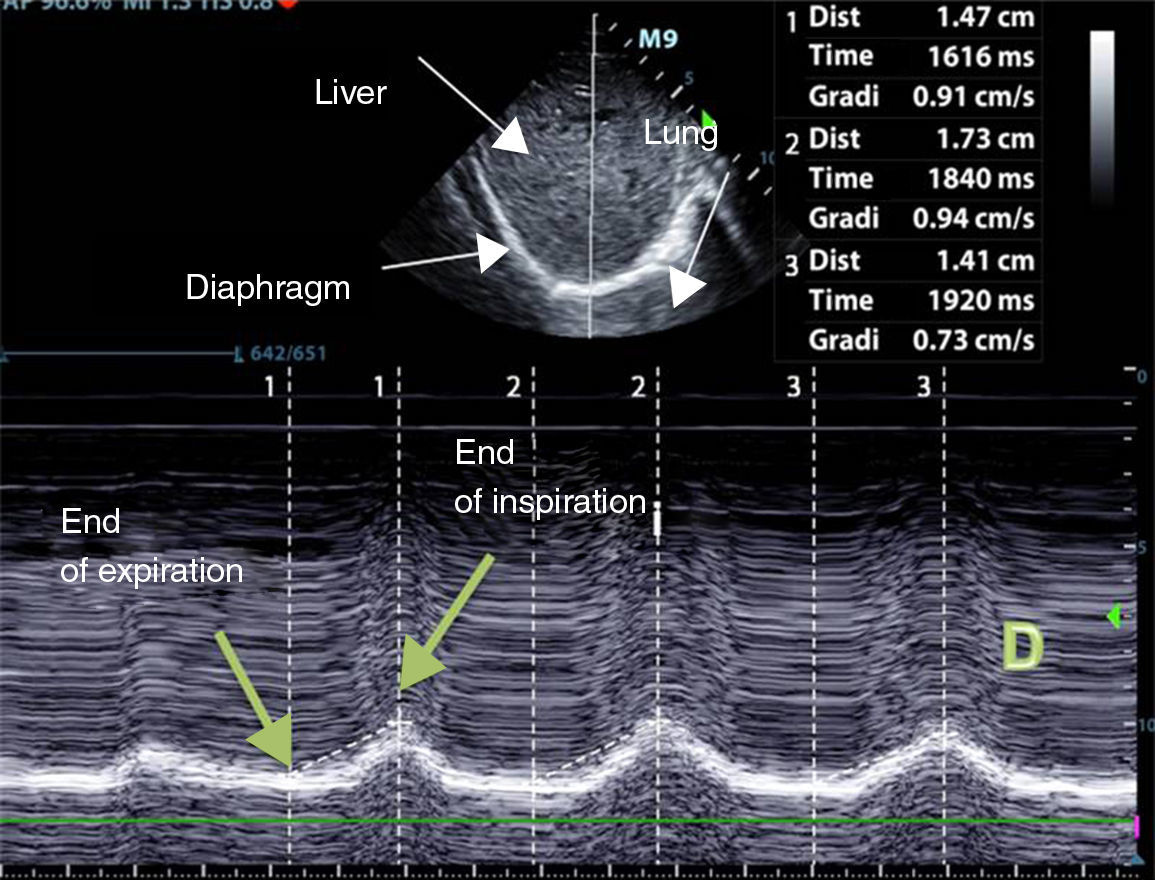
Diaphragm ExcursionTo evaluate diaphragm motion, a 3.5–5MHz probe must be positioned below the rib cage at the level of the mid-clavicular line, directing the ultrasound beam perpendicularly toward the posterior third of the hemidiaphragm. During inhalation, the normal diaphragm moves down, and thus approaches the transducer (Fig. 1).
Normal values in healthy non-ventilated patients differ between men and women (18±3 and 16±3mm, respectively),69 similarly to baseline values in mechanically ventilated patients.71 Diaphragm dysfunction is defined as an excursion of less than 10mm or a negative excursion (or paradoxical movement). These values are also good predictors of failure to wean.72
Diaphragm ThicknessDiaphragm thickness depends on muscle mass, and is correlated with forced vital capacity.73 Reduced diaphragm thickness is associated with low amplitudes on electromyography, and may indicate the presence of atrophy.23,74
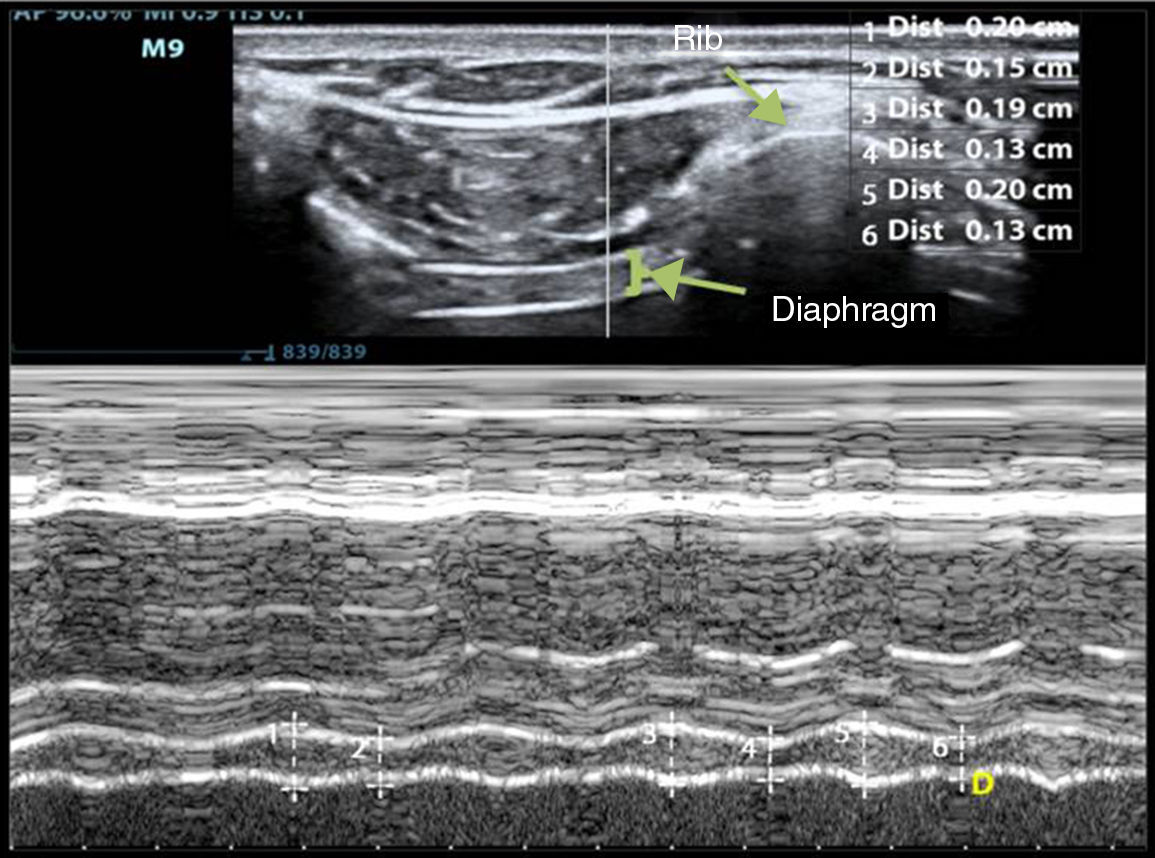
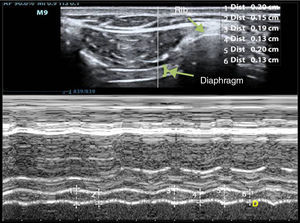
Diaphragm thickness can be quantified simply using ultrasonography, with the right hemidiaphragm, via the hepatic window, being more accessible than the left.51 To determine thickness, a 10–12MHz probe, positioned perpendicularly over the 9th–10th intercostal space in the anterior axillary line, is used to observe part of the zone of apposition of diaphragm to rib cage. In this area, the diaphragm is seen as 3 parallel layers of differing density (pleura, diaphragm, and peritoneum).51,70 Diaphragm thickness is measured by M-mode or with a 2D image during non-forced expiration (Fig. 2). Normal diaphragm thickness in ventilated patients is 2.4±0.8mm, with atrophy defined as values below 2mm.51 Ratios of 1.8 are considered normal, with a lower accepted limit of 1.2.68
M-mode measurement of diaphragm thickness variation of the right hemidiaphragm. The diaphragm is seen as 3 parallel lines: 2 hyperechogenic layers and a thicker, central hypoechogenic layer. Number 1 is the inspiratory thickness and number 2 is the expiratory thickness. Diaphragm shortening fraction is the difference between the inspiratory thickness and the expiratory thickness.
Variation in diaphragm thickness can be calculated using M-mode with the formula indicated in Fig. 3. Diaphragm thickness variation can be used as an indicator of diaphragm capacity to generate pressure.52,75 A variation of <20% may be considered as a predictor for failure to wean54 and is a better prediction than the Tobin index.76
Grosu et al. found a daily reduction of 6% in diaphragm thickness in the first 48h after starting ventilation with assisted modalities.67 In the study of Schepens et al., in which most patients were receiving CMV, a 10.9% loss of thickness was reported.71 Although no direct association was found between diaphragm thickness and CMV, the data show a greater loss of thickness in patients primarily ventilated using the controlled modality.
In MV patients, changes in diaphragm thickness, unlike diaphragm excursion, may really reflect active diaphragm contraction. This was demonstrated by Goligher et al., who did not observe variations in relaxed patients but did see changes in healthy volunteers and patients undergoing different MV methods.74 In non-ventilated patients, thickness variation has been shown to be directly related with lung volumes70,77,78 and with maximum inspiratory pressure79 and, as such, could be an indirect measurement of contractile activity and the diaphragm workload.
Laboratory MarkersNo relationship between the appearance of respiratory muscle atrophy and increased muscle enzymes, such as creatine kinase and myoglobin, has been observed in blood tests.9 An increase in skeletal protein troponin I may, in the future, be used as a marker of diaphragm muscle damage.80–82
ConclusionsDiaphragm dysfunction in the mechanically ventilated patient is a disorder that is still undefined and underdiagnosed. It appears early after the initiation of MV and is associated with risk factors such as sepsis and multiorgan failure. It affects a high percentage of patients and leads to extubation failure.
Future studies are needed to determine the initial processes that trigger protein changes and to develop non-invasive techniques, accessible at the bedside, in order to establish an early diagnosis: in this respect, ultrasonography is playing an increasingly important role.
Conflict of InterestsThe authors state that they have no conflict of interests.
We thank Mrs Marta Gas, from the Intensive Medicine Department of the Hospital del Mar, Barcelona, for her willingness and kind collaboration.
Please cite this article as: Dot I, Pérez-Teran P, Samper M-A, Masclans J-R. Disfunción diafragmática: una realidad en el paciente ventilado mecánicamente. Arch Bronconeumol. 2017;53:150–156.