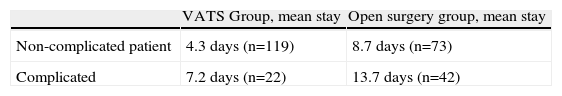Surgical treatment of stage I non-small cell lung cancer (NSCLC) can be performed either by thoracotomy or by employing video-assisted thoracic surgery (VATS). The aim of this study was to compare long- and short-term results of conventional surgery (CS) vs VATS lobectomy in the treatment of stage I NSCLC.
Materials and methodsWe performed a retrospective, analytical study of patients undergoing surgery for stage I NSCLC during the period January 1993 to December 2005. The variables analysed were overall survival, recurrence, distant metastasis, morbidity, mortality and hospital stay. During this period, 256 anatomic lung resections were performed: 141 by CS and 115 by VATS.
ResultsThere were statistically significant differences in: (i) mean hospital stay in patients with no complications (VATS group: 4.3 days vs CS group: 8.7 days, P=.0001); (ii) mean hospital stay in patients with complications (VATS: 7.2 days vs CS: 13.7 days, P=.0001), and (iii) morbidity (VATS: 15.6% vs CS: 36.52%, P=.0001). No statistically significant differences were found in: (i) mortality (VATS: 2.17% vs CS: 1.7%, P=.88); (ii) 5-year overall survival (VATS: 68.1% vs CS: 63.8%), and (iii) local recurrence and distant metastasis (P=.82).
ConclusionsVATS lobectomy is a safe and effective approach, with a shorter hospital stay and lower morbidity than CS; no statistically significant differences were observed in survival in patients undergoing surgery for stage I NSCLC.
Analizar nuestra experiencia con la cirugía torácica videoasistida (VATS) y comparar sus resultados a corto y a largo plazo con la lobectomía por cirugía convencional, en el tratamiento quirúrgico del cáncer de pulmón no microcítico (CPNM) en estadio I.
Material y métodosSe realizó un estudio retrospectivo y analítico de los pacientes intervenidos de cáncer de pulmón no microcítico en estadio I durante el periodo de enero de 1993 a diciembre de 2005. Las variables analizadas fueron: supervivencia global, recidiva, metástasis a distancia, morbimortalidad y estancia hospitalaria. Durante este periodo se realizaron 256resecciones pulmonares anatómicas: 141 por VATS y 115 por cirugía convencional.
ResultadosSe encontraron diferencias estadísticamente significativa en: a) estancia media postoperatoria en pacientes que no tuvieron complicaciones (grupo VATS: 4,3días; grupo de cirugía convencional: 8,7días; p=0,0001); b) estancia media postoperatoria en pacientes que tuvieron complicaciones (VATS: 7,2 días; cirugía convencional: 13,7 días; p=0,0001), y c) morbilidad (VATS: 15,6%; cirugía abierta: 36,52%; p=0,0001). No se encontraron diferencias estadísticamente significativas en: a) mortalidad (VATS: 2,17%; cirugía convencional: 1,7%; p=0,88); b) supervivencia global a 5años (VATS: 68,1%; cirugía convencional: 63,8%); c) recidiva local y metástasis a distancia (p=0,82).
ConclusionesLa lobectomía VATS es una técnica segura y eficaz, con una menor estancia hospitalaria y morbilidad que la cirugía convencional, sin que se observen diferencias estadísticamente significativas en la supervivencia en pacientes intervenidos por cáncer de pulmón no microcítico en estadioi.
During the last decade, lung cancer has been the principal cause of cancer death throughout the world and remains one of the respiratory diseases with the highest mortality rates: every year, 900000 new cases are diagnosed in males and 330000 in females. In the European Union, lung cancer accounts for 21% of all cancers in males and causes 29% of all cancer deaths in that gender.1
In 1993, Roviaro et al.2 reported the first successful lobectomy carried out with video-assisted thoracic surgery (VATS), demonstrating that lung cancer could be appropriately treated in this way while describing the surgical technique employed.
Since then, various authors have reported high numbers of both lobectomies and pneumonectomies performed using this technique,3–6 with better immediate outcomes when compared to open surgery. Nevertheless, lobectomies by thoracotomy continue to be carried out in patients with early stage lung cancer. The primary aim of our study is to compare the two approaches for the surgical treatment of stage inon-small cell lung cancer and to evaluate their outcomes, with the aim of defining the real advantages of VATS.
Materials and MethodsThis was a comparative, retrospective study in our centre, analysing the short and long-term outcomes of patients with a diagnosis of stage i non-small cell lung cancer undergoing major anatomical lung resection with systematic lymph node dissection. The TNM classification (6th edition) for lung cancer was used in all cases. One hundred and sixty-one (161) major lung resections were performed using VATS and 142 were undertaken using conventional surgery. Twenty (12.4%) cases in the VATS group and 27 (19%) in the open surgery group were lost to follow-up.
The clinical variables studied were conversion rates, duration of the intervention, mortality, number of complications, hospital stay after surgery (complicated and non-complicated patients), local recurrence rates, distant metastases and 5-year survival. Pearson's χ2-test and the Mantel–Cox method were used for analysis, with a value of P<.05 being considered statistically significant.
Characteristics of the Two Groups- -
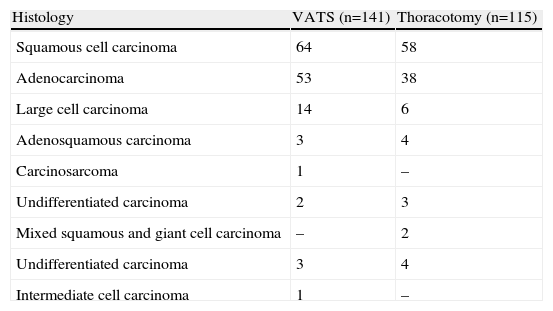
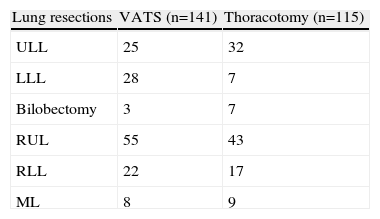
VATS group (n=141 patients; 102 men and 39 women). Mean age was 60.4 years (range 34–79 years). Histological types are listed in Table 1, squamous cell carcinoma being the most common (45.2%). The mean tumour size was 3.1cm (range 1.1–6.2cm). One hundred and thirty-eight (138) lobectomies and 3 bilobectomies were performed (Table 2).
Table 1.Histological Types.
Histology VATS (n=141) Thoracotomy (n=115) Squamous cell carcinoma 64 58 Adenocarcinoma 53 38 Large cell carcinoma 14 6 Adenosquamous carcinoma 3 4 Carcinosarcoma 1 – Undifferentiated carcinoma 2 3 Mixed squamous and giant cell carcinoma – 2 Undifferentiated carcinoma 3 4 Intermediate cell carcinoma 1 – - -
Conventional surgery group (n=115 patients; 88 men and 27 women). Mean age was 62.6 years (range 37–83 years). As in the VATS group, the most common histological type was squamous cell carcinoma (50.7%) (Table 1). The mean tumour size was 3.6cm (range 1.4–6.8cm). One hundred and eight (108) lobectomies and 7 bilobectomies (Table 2) were performed.
Homogeneity between both groups was tested statistically with regard to sex, age, histological type, tumour size and resection type, using Snedecor's F distribution.
Selection CriteriaInclusion criteria. All patients with a diagnosis of stage i non-small cell lung cancer (NSCLS) resected between 1 January 1993 and 31 December 2005; thus, all patients would be in follow-up for a minimum of 5 years.
Exclusion criteria. Minor lung resections (atypical resection or segmentectomy) or major anatomical resection with pneumonectomy, sleeve lobectomy, bronchoplasty or broncho-angioplasty. Cases in the VATS group which required conversion to conventional surgery (24) once dissection had begun were also excluded.
Selection of approach. The choice of an open approach or VATS was a decision made individually by each surgeon after exploratory video-assisted thoracoscopy, although since that time the criteria in our department have been unified. For lung resection to be performed using VATS, the cases had to meet the following criteria:
- 1.
Tumour <4cm. This is the ideal size, although in our study, tumours of up to 6cm were successfully resected, as there is usually no problem if the location is sufficiently peripheral.
- 2.
The tumour had to be peripheral, and never in the lobar bronchi, or at least 2cm from the interlobar carina.
- 3.
Open fissure, although this is now in question. On the right side, the minor fissure does not present any problem in upper, mid and lower lobectomies, and fused major fissure is not a problem, neither for an upper lobectomy nor for a lower lobectomy, where the bronchus must be done before the artery. On the left side, the major fissure must be open, although in cases where the fissure is fused, the lobar bronchus may be done first, as on the right side.
- 4.
Pleural adhesions are a relative contraindication for VATS, since they only represent a real obstacle when they are firm and extensive; otherwise they can generally be completely freed.
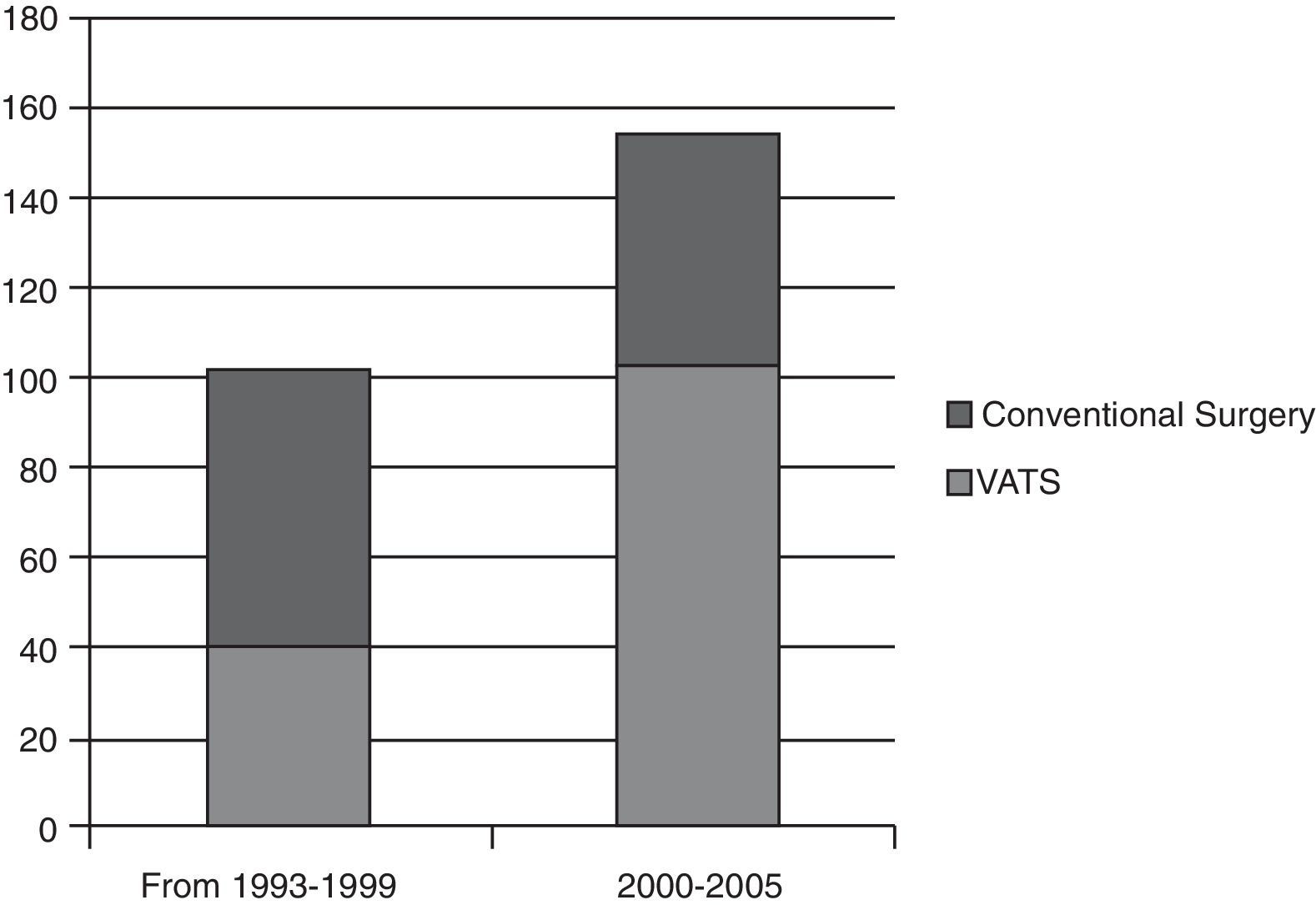
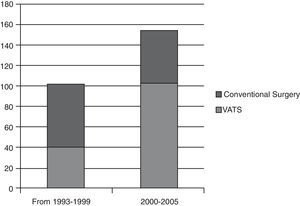
In our study, anatomical lung resections were performed in 256 patients with stage i non-small cell lung cancer. If the number of VATS lung resections carried out in the first six years (1993–1999) is compared with the last five years of the study (Fig. 1), a significant increase can be observed in the number of VATS lobectomies (37.5%) compared to lobectomies by conventional surgery. This rise can be attributed to our surgical team overcoming the learning curve and a reduction in the number of contraindications for VATS as our experience grew. For example, at the beginning, pleural adhesions were a contraindication for this type of surgery, but now open surgery is undertaken in only a small number of cases. The rate of conversion to open surgery in our study is 14.5% (n=24). The reason for conversion was due to controlled bleeding in 13 cases and in the others it due to technical difficulties (multiple and/or firm adhesions, calcified lymph nodes, oncological reasons, anaesthetists could not achieve adequate lung collapse). Notwithstanding, on examining the number of conversions in the last 5 years, the conversion rate has fallen to 7.6%.
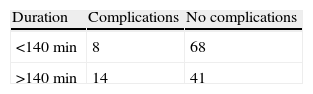
It is also important to determine the time required for a VATS lung resection and if this impacts on the development of complications. If surgical time is taken as the time from making the first port incision until closure, the mean duration in the VATS group was 138.3min (median 92min). When surgical time is correlated with development or non-development of complications, using Pearson's χ2 test, a prevalence ratio of 2.42 (95% CI, 1.09–5.36, P=.043) is obtained, showing that surgical time is a risk factor for the development of post-operative complications (Table 3). The mean duration of conventional surgery was 126.6min (median 123min).
Morbidity and MortalityLet us first examine mortality in the first 30 days (Table 4). Pearson's χ2 test gave a P-value of .88, showing that the risk of death after conventional thoracotomy is no greater than with VATS.
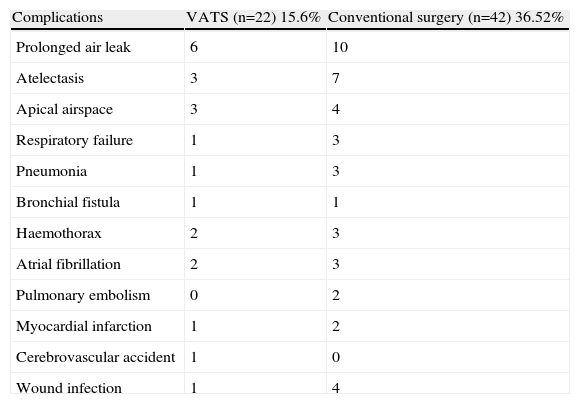
The overall number of complications that emerged in our series was 64, of which the most frequent were pulmonary (48; 75%), followed by cardiovascular (10; 15.6%), surgical wound problems (5; 7.8%) and cerebrovascular accident (1; 1.6%).
Overall morbidity in the conventional surgery group was greater than in the VATS group (Table 5), due to greater trauma to the chest wall and the lung caused by thoracotomy. In view of these data, a Pearson's χ2-test was performed on the number of patients who underwent resection in order to determine whether the approach adopted affected the development of complications in the post-operative period. The P-value was .0001, with a prevalence ratio of 0.55 (95% CI, 0.39–0.79), showing a significant association between the approach used and the rate of complications.
Morbidity in the Two Groups.
| Complications | VATS (n=22) 15.6% | Conventional surgery (n=42) 36.52% |
| Prolonged air leak | 6 | 10 |
| Atelectasis | 3 | 7 |
| Apical airspace | 3 | 4 |
| Respiratory failure | 1 | 3 |
| Pneumonia | 1 | 3 |
| Bronchial fistula | 1 | 1 |
| Haemothorax | 2 | 3 |
| Atrial fibrillation | 2 | 3 |
| Pulmonary embolism | 0 | 2 |
| Myocardial infarction | 1 | 2 |
| Cerebrovascular accident | 1 | 0 |
| Wound infection | 1 | 4 |
The last important point in this comparison concerns the mean post-operative hospital stay after both procedures, which is basically determined by morbidity. To this end, the mean number of days of the post-operative stay were evaluated according to the approach used, by complicated and non-complicated patients (Table 6). The Pearson's χ2 test was applied, giving a highly significant P-value (P=.0001) and a prevalence ratio of 0.21 (95% CI, 0.15–0.31).
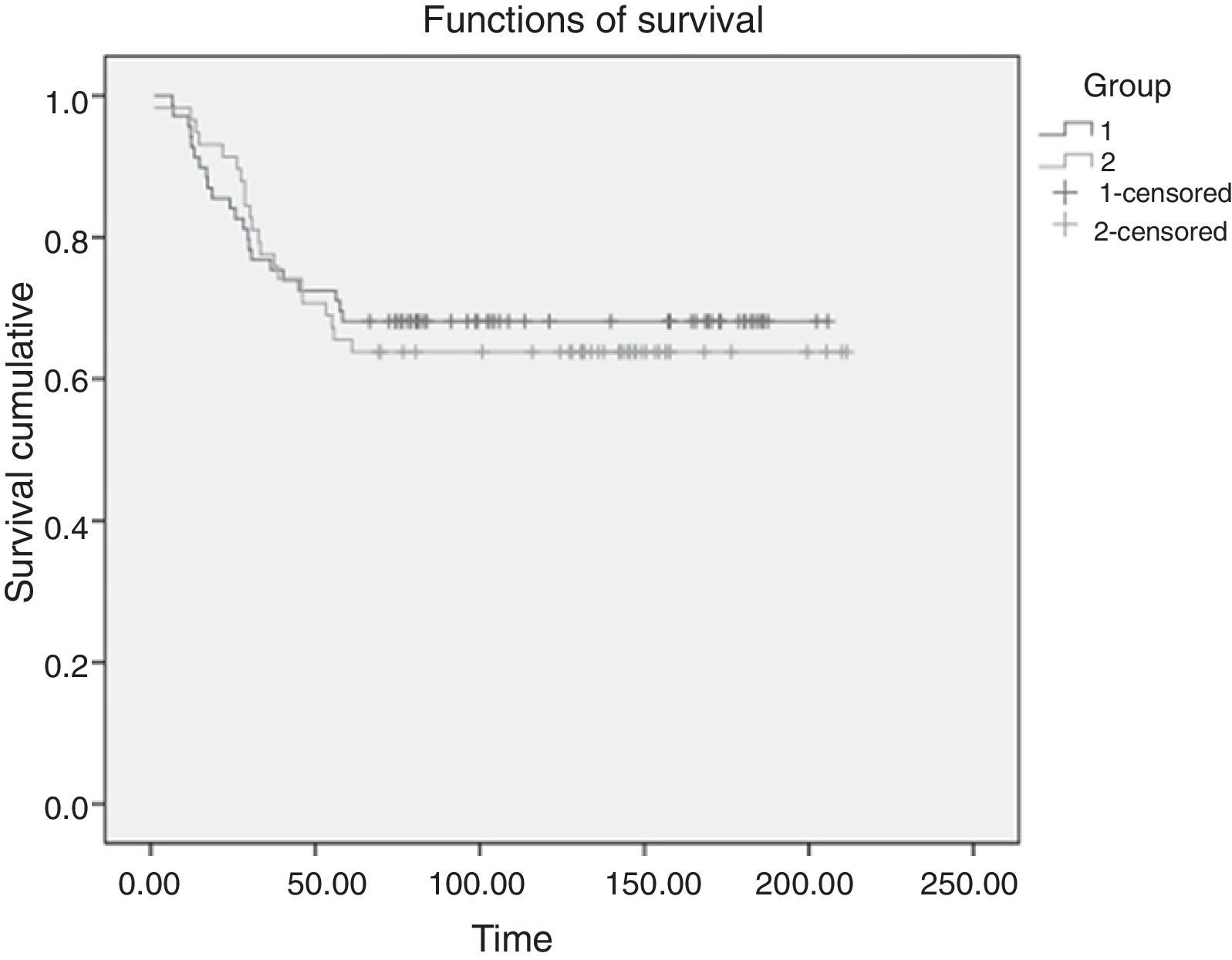
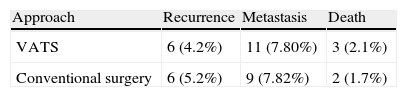
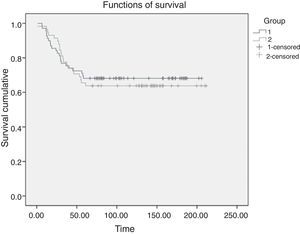
SurvivalThe 5-year survival rate after the intervention was 68.1% in the VATS group and 63.8% in the conventional surgery group. A Mantel–Cox non-parametric test that accepted censored data was used to compare both survival curves (Fig. 2). No statistically significant differences were observed in overall survival (log-rank test, P=.735).
Recurrence Rate and Distant MetastasesNo statistically significant differences (P=.84) were observed when the recurrence rates of patients who underwent resection using conventional surgery and VATS were compared (Table 4). The relationship between the development of distant metastases and the approach is not statistically significant (Table 4), according to a Pearson's χ2 test (P=.82).
DiscussionThe first successful lobectomy with VATS was carried out in 1993.2 Several advantages of this technique over conventional surgery have been subsequently demonstrated. In our study, in order to make a comparison between the two approaches and to assess the outcomes, patients with stage i (TNM) lung cancer were selected to undergo lobectomy/bilobectomy by VATS or open surgery. Mean tumour size in both groups was practically the same (VATS: 3.1cm; open surgery group: 3.6cm) and the mean age of both groups was similar. Accordingly, any influence derived from the stage, tumour size, type of resection or age of the patients on surgical outcomes and long-term survival was avoided.
As in other studies,6,7 morbidity in the VATS group (15.6%) was lower than that in the open surgery group (36.52%) (P=.0001). However, in a study8 comparing 122 patients undergoing VATS lobectomy with 122 undergoing conventional surgery, no statistically significant differences were observed when the different types of complications were analysed individually. This is probably because in that study the groups were classified by sex and age, without taking into account other characteristics including stage, tumour size, etc. These factors were included in the series published by Villamizar et al.,7 in which 284 VATS patients were compared with 284 conventional surgery patients on the basis of 13 perioperative variables. There were fewer complications in the VATS group (21%) than in the conventional surgery group (49%). In our series, pulmonary complications were the most common, and only 1.4% of the VATS group and 2.6% of the thoracotomy group experienced atrial fibrillation in the post-operative period. Morbidity was lower because VATS is less aggressive to the chest wall and lung and has less impact on the breathing mechanism, unlike thoracotomy, which produces more cases of atelectasis, pneumonia, prolonged air leak, etc.
Obviously, the lower rate of complications in the VATS group will impact positively on the mean hospital stay; in our study the difference between the two approaches was highly significant (P=.0001), both in the group of complicated patients and in the patients without complications. This reduction in the length of hospital stay in the VATS group has been described in other studies,6,7,9 and attributed to less post-operative pain or the early withdrawal of pleural drainage.
As in the larger series published,4,6–13 which show mortality ranging between 0.4% and 3.7%, in our study the mortality rate in the first 30 days post-surgery in the VATS group was very low. Similarly to our series, no differences were found between the two groups in the first 30 days post-surgery in the prospective study performed by Villamizar et al.,7 showing that VATS did not increase the risk of death.
The mean duration of a VATS lobectomy in a clinical trial performed by the American College of Surgeons Oncology Group Z00309 was 117minutes, and 171minutes by lateral thoracotomy (P=.001). However, in the study of Subroto et al.,10 centred on the Society of Thoracic Surgeons database, the duration of a VATS lobectomy (173min) was greater than that by the conventional method (143min), with a statistically significant difference (P=.0001). In our series, the duration of a VATS lobectomy (median: 92min) did not involve longer surgery time or more time under anaesthesia. In addition, if surgical time is compared with the development or non-development of complications, the duration of the intervention (>140min) is found to be a risk factor for development of post-operative complications (P=.043). These results suggest that although the aggression to the chest wall and the resulting repercussions on post-operative progress after one approach or another are factors to be taken into account, the length of time in surgery must also be taken into consideration.
Despite the advantages offered by anatomical lung resections with video-assisted surgery, its use in the treatment of lung cancer continues to be controversial. The defenders of the technique, including our team, maintain that in a VATS lung resection all the principles of oncological surgery are respected, including complete resection (R0) and complete lymph node dissection. Although the role of VATS in performing complete lymph node dissection has been questioned, in our experience all lymph node stations are equally or more accessible using this technique when compared to thoracotomy, and is therefore perfectly valid and safe for this purpose. In the randomised study by Kirby and Rice,5 no statistically significant differences were observed between the total number of lymph nodes obtained in the two approaches (total number of lymph nodes from VATS: 9.5±3.6; from thoracotomy: 9.3±4.3). In another study by Sagawa et al.,12 in which a thoracotomy was performed after VATS lobectomy in order to verify whether complete lymph node dissection had been achieved, the number of remnant nodes after a VATS lymphadenectomy was between 2% and 3% (on the right side an average of 1.3 nodes of a mean total of 40.3 nodes was found, and on the left side, 1.2 of a mean total of 37.3).
Detractors of VATS point to another randomised study in which no particular advantage was seen for this technique, since the patients had similar hospital stays and post-operative morbidities with both approaches.13 They also point out that most of the series published in favour of VATS were retrospective case-control studies.11 However, the prospective, randomised, multicentre study carried out by Scott et al.9 with a scoring system for obtaining two groups with similar baseline characteristics, showed a shorter hospital stay and a lower number of complications in the VATS group. Similarly, the morbidity rate in the VATS group in our series was lower than in the open surgery group (P=.0001), as was the mean post-operative stay in complicated and non-complicated patients (P=.0001).
Another aspect showing that VATS complies with the principles of oncological surgery is the recurrence rate and overall survival. In our study, follow-up was at least 5 years and no statistically significant differences were found in overall survival (log-rank test, P=.735), although our results suggested a slight increase in survival in the VATS group. Similarly, no statistically significant differences were observed in the number of recurrences and distant metastases on comparing the two approaches (P=.82). As in our results, the recent meta-analysis of 21 studies by Yan et al.11 found the same rate of recurrence after VATS lobectomies as after conventional surgery (RR: 0.64; 95% CI, 0.35–1.35; P=.24). The authors suggest that VATS lobectomies for lung cancer treatment are associated with a low rate of systemic recurrences (RR: 0.57; 95% CI 0.3–0.95; P=.03) and a better 5-year survival rate (RR: 0.66; 95% CI, 0.45–0.97; P=.04).
Although our study shows that video-assisted surgery is a safe and appropriate approach in patients with stage i NSCLC that complies with the principles of oncological surgery, the results should be evaluated with caution. Although the aim was to select a sample with similar clinical characteristics in both groups (age, sex, tumour size, TNM), this is a retrospective study, so there could be a risk of bias in the collection of the clinical data. Despite this, the use of this technique in the surgical treatment of NSCLC continues to increase, as demonstrated by the results obtained in the series published by Subroto et al.,10 Villamizar et al.7 and Scott et al.,9 who conclude that VATS lung resection for the treatment of NSCLC is a safe and effective treatment with fewer complications than open surgery and better outcomes with regard to hospital stay and aesthetic appearance. Long-term survival with both techniques is similar, although as surgeons’ experience increases and the number of cases of resection continues to rise, it seems that survival in the VATS group may be longer.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Triviño A, Congregado M, Loscertales J, Jiménez-Merchán R, Pinos-Vélez N, Cózar F, et al. Experiencia y desarrollo de la técnica de lobectomía por cirugía torácica videoasistida: estudio comparativo con cirugía convencional en estadio I de cáncer de pulmón no microcítico. Arch Bronconeumol. 2014;50:57–61.