Dyspnoea is a complex, highly personalised and multidimensional sensory experience, and its underlying cause and mechanisms are still being investigated. Exertional dyspnoea is one of the most frequently encountered symptoms of patients with cardiopulmonary diseases, and is a common reason for seeking medical help. As the symptom usually progresses with the underlying disease, it can lead to an avoidance of physical activity, peripheral muscle deconditioning and decreased quality of life. Dyspnoea is closely associated with quality of life, exercise (in)tolerance and prognosis in various conditions, including chronic obstructive pulmonary disease, heart failure, interstitial lung disease, and pulmonary hypertension, and is therefore an important therapeutic target.
Effective management and treatment of dyspnoea is an important challenge for caregivers, and therapeutic options that attempt to reverse its underlying cause have been only partially successful This “review” will attempt to shed light on the physiological mechanisms underlying dyspnoea during exercise and to translate/apply them to a broad clinical spectrum of cardio-respiratory disorders.
La disnea es una experiencia sensorial compleja, multidimensional y muy personal cuyo origen y mecanismos todavía se están investigando. La disnea de esfuerzo es uno de los síntomas más frecuentes de los pacientes que padecen enfermedades cardiopulmonares y un motivo habitual que les impulsa a buscar atención médica. El síntoma progresa de forma implacable a medida que la enfermedad avanza y conduce al paciente a evitar la actividad, con la consecuente atrofia de la musculatura periférica y pérdida de calidad de vida. La disnea guarda una estrecha relación con la calidad de vida, la (in)tolerancia al ejercicio y el pronóstico de diversas patologías, que incluyen la enfermedad pulmonar obstructiva crónica, la insuficiencia cardíaca, la enfermedad pulmonar intersticial y la hipertensión pulmonar, por lo que es un objetivo terapéutico importante.
El manejo y tratamiento eficaces de la disnea suponen un importante desafío para los cuidadores, y las opciones terapéuticas que pretenden revertir la causa subyacente solo han sido satisfactorias hasta cierto punto. En esta «revisión» intentaremos desvelar los mecanismos fisiológicos de la disnea de esfuerzo y traducirlos o aplicarlos a una amplia gama clínica de patologías cardiorrespiratorias.
Dyspnoea is worth documenting and accurately assessing for at least the following reasons: (1) exertional dyspnoea is frequently one of the earliest and more troublesome symptoms that lead patients with chronic cardiac or pulmonary illnesses to seek medical care; (2) it usually progresses with the severity of the underlying disease, often leading to a vicious circle characterised by avoidance of physical activity leading to muscle deconditioning and decreased quality of life; (3) up to half of patients with chronic diseases are affected by it; (4) dyspnoea is closely associated with quality of life, exercise (in)tolerance and prognosis in various disease conditions, including chronic obstructive pulmonary disease (COPD), in which it is known to predict mortality with greater accuracy than forced expiratory volume in 1s (FEV1), and chronic heart disease, in which dyspnoea is more closely associated with mortality than the presence of angina; (5) it is an important factor in the low adherence to exercise programmes in various populations, including patients with COPD. Effective management and treatment of dyspnoea is a major challenge for caregivers, and therapeutic options that attempt to reverse its underlying cause have been only partially successful.1–7
Statements from the American Thoracic Society8 and European Respiratory Society1 have drawn attention to the multidimensional nature of dyspnoea, which involve three main domains: (1) the sensory-perceptual domain, (2) affective distress, and (3) the symptom impact domain.
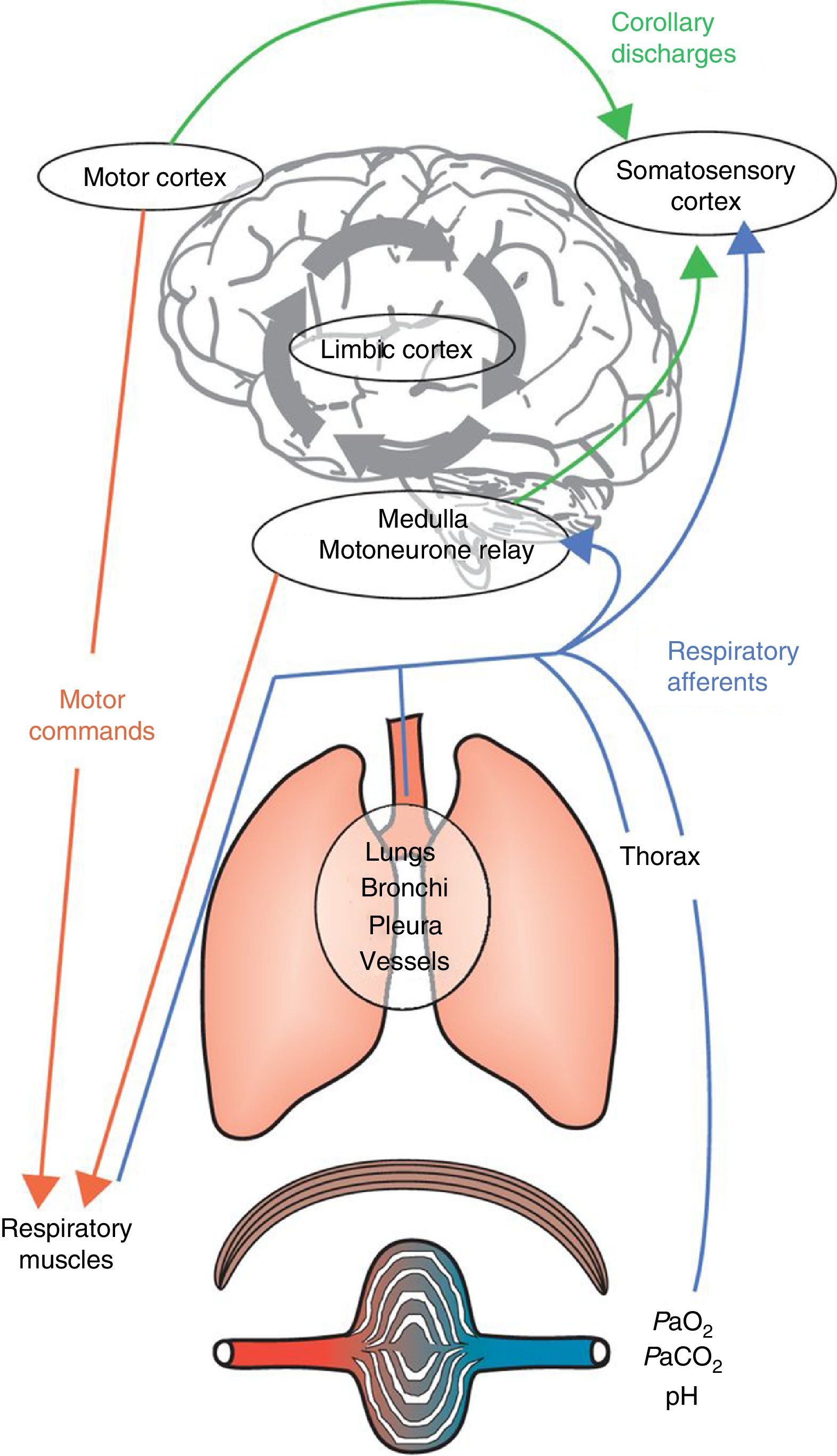
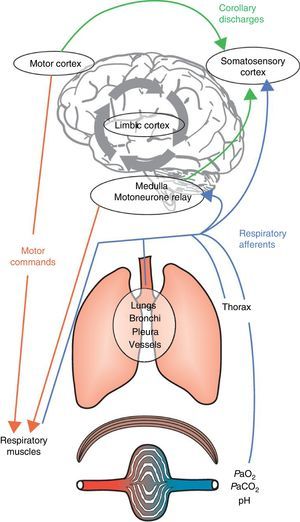
Dyspnoea is a complex and highly personalised experience, the cause and mechanisms of which are still incompletely understood. Dyspnoea is felt when there is a conscious awareness of the dissociation between the expectations of the brain (through the “corollary discharge”, i.e., a copy of the descending motor activity sent by respiratory motor areas of the brain to perceptual areas) and the neural inputs it receives from the respiratory system, respiratory muscles, peripheral chemoreceptors and locomotor muscles.8 Not all conscious breathing sensations are labelled “dyspnoea” because the brain is able to “filter” most afferent respiratory inputs and allow only a fraction to reach consciousness.1 This sensory gating mechanism allows the brain to distance itself from irrelevant or sensory data. Human beings have the capacity to voluntarily bring “breathing” into awareness at any moment. Alternatively, breathing can become impossible to ignore if it requires immediate attention, a sensation usually described as dyspnoea. This “gating process” is instrumental in monitoring essential biological and physiological functions and adopting appropriate behaviour. The neural gating of respiratory afferent input into the cerebral cortex will create a cognitive awareness of breathing and an emotional or affective response.1 To summarise, dyspnoea may result from two interrelated mechanisms: (1) a discriminative one, which identifies relevant afferent information on respiratory disruption/abnormalities and brings them into consciousness (sensory components: intensity and quality); and (2) an affective one which labels them as unpleasant or threatening (Fig. 1).
Integrative mechanisms underlying dyspnoea. Respiratory command derives from inputs from both the motor cortex and the medulla. These commands are integrated at the spinal level and transmitted to the muscular effectors of the respiratory system. The subsequent activation of the respiratory muscles will generate afferent inputs that are fed back to the respiratory command centres and the somatosensory cortex. The comparison of the corollary discharge and the ensuing afferent feedback may cause a mismatch, and dyspnoea will occur when a negative affective sensation is attributed to this mismatch by the limbic cortex, which will also be influenced and modulated by memory and the prevailing environment.
Adapted from Laviolette L, Laveneziana P. The European Respiratory Journal. June 2014;43(6):1750–1762, with permission.
Dyspnoea can also be quantified (“intensity”). Exertional dyspnoea can be seen as “the perception of respiratory discomfort that occurs for an activity level that does not normally lead to breathing discomfort”. It follows that the intensity of dyspnoea can be determined by assessing the activity level required to produce dyspnoea.1
Dyspnoea can therefore be evaluated during a physical task, such as cardiopulmonary exercise testing (CPET).1,8 For this purpose, the 10-point Borg scale can be used to rate a specific respiratory sensation (e.g., inspiratory difficulty, breathing effort, expiratory difficulty, air hunger, etc.) or a more general one (e.g., breathing difficulty, breathlessness). Though somewhat less popular, the visual analogue scale (VAS) is another dyspnoea measuring instrument during CPET, with proven construct validity used. Both the VAS and Borg scale have been shown to provide similar scores during CPET, and to be reliable and reproducible over time in both healthy subjects and patients with chronic respiratory diseases undergoing CPET.9 The advantage of using the Borg or VAS scales in individual patients is the possibility of reliably comparing “intensity of exertional dyspnoea” at the same level of exercise intensity (standardised work-rate or oxygen consumption or minute-ventilation) between subjects, and evaluating the response to a therapeutic intervention.9 Studies have shown that there is a close correlation between the intensity of the work of breathing (measured by the ratio of tidal oesophageal pressure relative to its maximal value) and the severity of dyspnoea during CPET, and that pharmacological interventions that decrease the magnitude (and length) of breathing efforts are associated with decreased dyspnoea intensity.1,9
Can Quality and Quantity of Dyspnoea be Assessed Simultaneously?The nature and intensity of dyspnoea can be measured together using CPET. Studies have shown that patients with chronic obstructive pulmonary disease (COPD) and mild stable asthmatics are able to “perceive” dynamic changes in their mechanics of breathing during exercise. When a critical value of inspiratory reserve volume (IRV) is attained (0.3–0.5L short of total lung capacity), tidal volume is constrained, dyspnoea intensity increases rapidly and there is a change in the main qualitative descriptor choice from work/effort to difficult/unsatisfied inspiration.1 The clinical importance of this phenomenon is that an evaluation of the quality of dyspnoea at the end of an exercise session can show whether a critical IRV (difficult/unsatisfied inspiration) has been attained or not (work/effort).1 CPET allows an understanding of the mechanisms underlying dyspnoea during exercise (“exertional dyspnoea”). CPET may also help clinicians identify additional mechanisms underpinning greater dyspnoea intensity which could be “independent of” or “not directly related to” the main/obvious pathophysiological determinant of the disease under consideration.
Affective and Emotional ComponentsBreathing sensations carry affective components that can be affected by and/or change irrespective of the sensory qualities of dyspnoea. In this sense, pain and dyspnoea are similar in terms of their multidimensional aspects.1,8 In healthy subjects breathing against inspiratory resistive loads, the level of unpleasantness of the breathing sensations increase more than its level of intensity, indicating that the sensory and affective facets of dyspnoea can be differentiated in a similar manner to the same aspects of pain.10Air hunger, meanwhile, is more often associated with unpleasantness and a negative emotional component than work/effort.
Scales that evaluate breathing discomfort by incorporating both intensity ratings and affective or emotional descriptors have been developed and validated in healthy subjects and those with chronic respiratory diseases, such as the Multidimensional Dyspnoea Profile11–13 and the Dyspnoea-12.14–17
Symptom Impact or BurdenThe symptom impact/burden component measures how dyspnoea/breathlessness affects functional ability, employment (disability), quality of life, or health status.1,8 Measurements include: (1) unidimensional rating of disability or activity limitation (e.g., MRC scale); (2) unidimensional or multidimensional rating of functional ability; (3) multidimensional scales of quality of life/health status. Questionnaires may include: Baseline Dyspnoea Index-Transition Dyspnoea Index (BDI-TDI), Chronic Respiratory Questionnaire, St. George's Respiratory Questionnaire.1,8
From Physiology in Health…Dyspnoea Can be Perceived as a Sensation of Respiratory EffortDuring voluntary increase in ventilation, the motor cortex increases the outgoing motor signal to respiratory muscles and sends a copy (central corollary discharge) through cortical interneurons to the sensory/association cortex, which is informed of the voluntary effort to increase ventilation.18 It is also likely that the sensation of respiratory effort arises from the simultaneous activation of the sensory cortex and muscle contraction: a variety of muscle receptors provide feedback on force and tension to the central nervous system, and information from these receptors may conceivably underlie the sensation of effort.18 For clinical purposes, the perceived magnitude of respiratory effort is expressed as the ratio of the tidal oesophageal pressure (Poes) to the maximal pressure generation capacity of the respiratory muscles (PI,max).18 In healthy subjects, volitional respiratory effort is matched with lung/chest wall displacement [i.e., change in tidal volume (VT) as a percentage of vital capacity (VC)] via concurrent afferent proprioceptive information, transmitted via vagal, glossopharyngeal, spinal, and phrenic nerves, that monitors displacement and is processed and integrated in the sensory cortex. The result is a harmonious neuromechanical coupling with avoidance of respiratory discomfort or distress.1,8,18
Dyspnoea Can be Perceived as a Sensation of Air HungerUnder some clinical and experimental circumstances the relationship between dyspnoea and effort is less apparent. If normal subjects suppress their ventilation to a level below that dictated by chemical drive (CO2), dyspnoea increases without a corresponding increase in respiratory effort.1,8,18 Likewise, in experimental and clinical conditions where peripheral stretch receptors are inhibited, the sensory cortex is not informed of the ventilatory response.18 In these circumstances, dyspnoea is perceived as a sensation of air hunger whose intensity depends on the mismatch between the level of chemical stimulated drive and the ongoing inhibition from pulmonary mechanosensors signalling the current level of ventilation.1,8,18 In turn, dyspnoea arises and may qualitatively change when peripheral afferent feedback is altered and inspiratory motor output either increases or stabilises.1,8,18
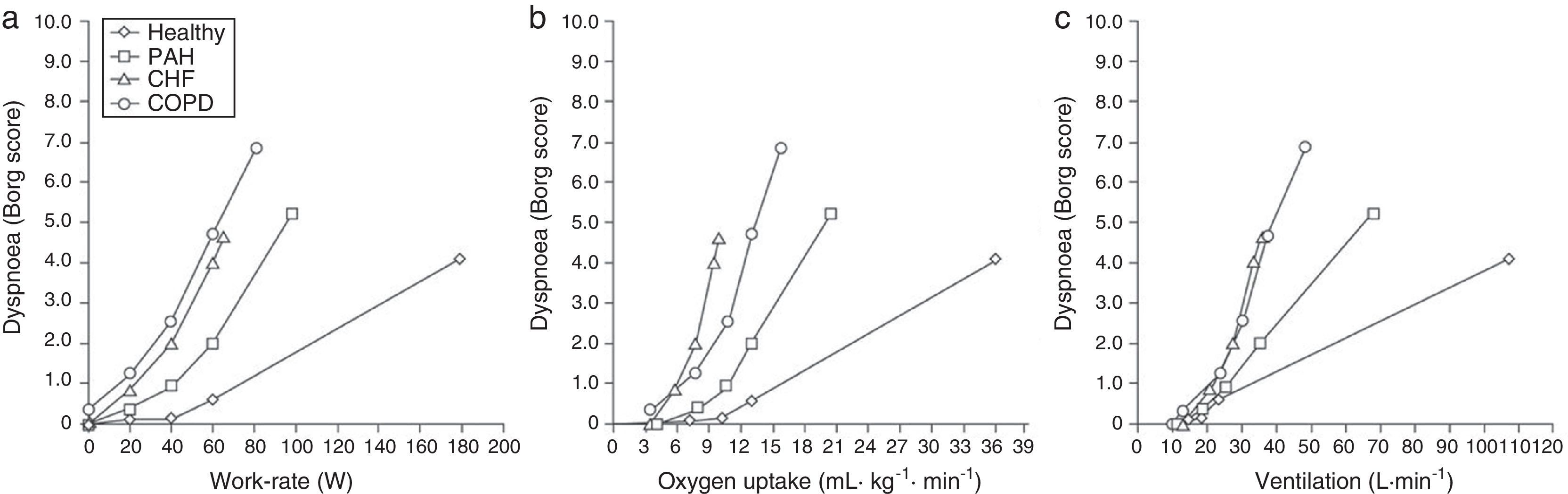
Dyspnoea on Exertion in Healthy, Non-athletic SubjectsDuring exercise, respiration adapts to the requirements of the muscular and cardiovascular systems. In healthy, nonathletic subjects, the reserve of the respiratory system is usually preserved and exercise is limited by reaching the maximal capacity of either the cardiovascular or the locomotor systems. Healthy subjects, therefore, usually report limb muscle fatigue as the primary cause for exercise cessation. A notable exception is high-level athletes, who may develop respiratory limitation to exercise1 because of exceptional cardiovascular or muscular performance. Dyspnoea in healthy subjects increases proportionally to minute-ventilation, which is dependent on respiratory contractile effort. This effort can be measured as the ratio of tidal oesophageal pressure swing to maximal pressure (see above). Healthy young adults generally rate dyspnoea intensity at the peak of exercise as “moderate” or “severe” (Fig. 2) and describe a heightened sense of effort, “work” or “heaviness” of breathing.1
Dyspnoea on exertion (rated using the Borg scale) with increasing work rate (left panel), oxygen uptake (centre panel) and minute-ventilation (right panel) in healthy subjects and patients with COPD, chronic heart failure and pulmonary arterial hypertension. Data from the authors’ laboratory in Paris, France. Adapted from Laviolette L, Laveneziana P. The European Respiratory Journal. June 2014;43(6):1750–1762, with permission.
Such sensations are not usually associated with a negative emotional response in this population, because they represent the expected ventilatory response to strenuous exercise.1 If the mechanical activity of the ventilatory system is adequate for the level of central respiratory motor drive, dyspnoea intensity increases in proportion to the level of neural respiratory drive, and is then described as a heightened sense of effort, work or heaviness of breathing.1,8
… to Clinical Implication in Cardio-Respiratory DisordersIn patients with respiratory and cardiovascular disorders, a ventilatory limitation of exercise is more frequent, although peripheral limb fatigue can also contribute to exercise limitation. For a given level of work, oxygen uptake or minute-ventilation, dyspnoea in patients with cardio-respiratory diseases is usually greater than in aged-matched controls (Fig. 2). In the following sections we will explore its underlying mechanisms.
COPDTwo main qualitative descriptor clusters of dyspnoea are frequently chosen by patients with COPD during physical activity.19–22 The descriptor cluster that alludes to increased respiratory work/effort (“breathing requires more effort or work”) is commonly selected by patients with COPD. Increased sense of work/effort is related to increased motor drive to the respiratory muscles and increased central neural drive (due to chemo-stimulation) as a consequence of progressive metabolic and ventilation/perfusion disruptions during exercise. Therefore, increased perceived work/effort during physical activity in part reflects the greater ventilatory demand when performing a given task compared with healthy individuals. In addition, contractile muscle effort is increased for any given ventilation because of: (1) acutely increased intrinsic mechanical (elastic/threshold) loading, and (2) functional respiratory muscle weakness. These respiratory mechanical/muscular abnormalities are, in part, related to resting and dynamic hyperinflation during exercise, and may lead to either a decrease in PI,max or a further increase in Poes as a percentage of PI,max. Because of these effects, greater neural drive or electrical activation of the respiratory muscle is required to generate a given force. Furthermore, because of the limbic system activation, the corollary discharge may be sensed as abnormal, thus evoking a sensation of distress.
The other descriptor cluster alludes to unsatisfied inspiration. Structural abnormalities (chronic bronchitis and emphysema), via their physiological negative consequences, i.e., expiratory flow limitation and dynamic hyperinflation, result in dyspnoea.23 Ventilation at high lung volumes has important negative mechanical and sensory implications. Dynamic lung hyperinflation forces tidal volume (VT) to be accommodated only within the inspiratory reserve volume, which becomes progressively reduced, and in the upper non-linear extreme of the contracted pressure–volume relationship of the respiratory system. The elastic loading of inspiratory muscles can be substantial in this situation. As lung hyperinflation develops, the inspiratory muscles must overcome the combined inward recoil of the lung and chest wall at the onset of inspiration, before the inspiratory flow commences. Dynamic lung hyperinflation shortens the operating length of the inspiratory muscles, thereby affecting their ability to generate pressure. The combination of excessive elastic and resistive loading results in functional muscle weakness, and the work required by the respiratory muscles to maintain tidal ventilation increases sharply. The net consequence is that the ratio of oesophageal pressure (expressed as a percentage of maximal inspiratory pressure) to VT [expressed as a percentage of TLC or vital capacity (VC)] increases significantly. One of the main consequences of hyperinflation is the constraint imposed on VT expansion: thoracic displacement is reduced (the VT response relative to TLC or VC diminishes or even reaches a discernable plateau) despite the almost maximal motor output generated by the respiratory muscles. This neuromechanical or neuroventilatory dissociation caused by dynamic hyperinflation is one of the main contributors to dyspnoea in patients with COPD.1,19–24
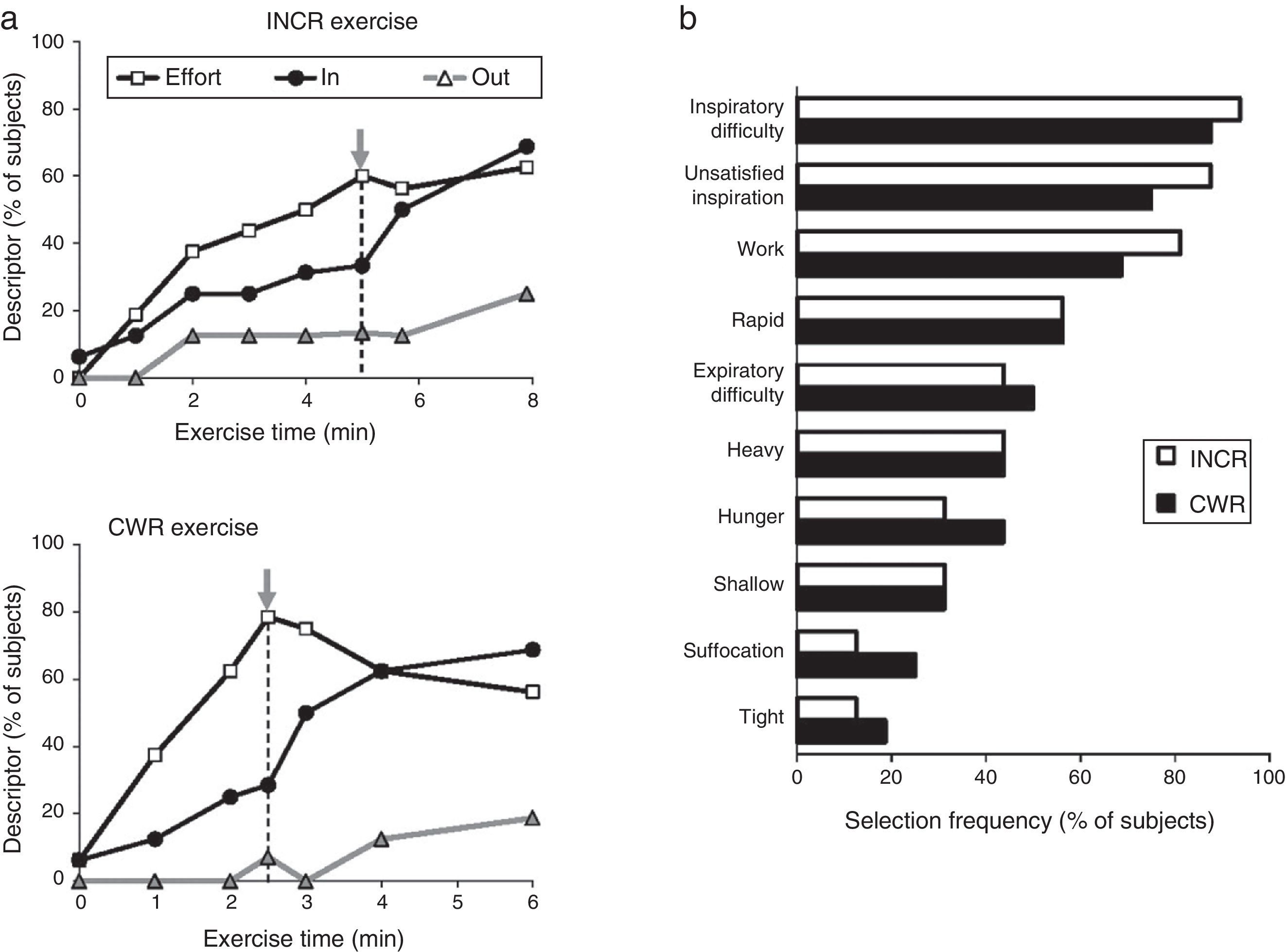
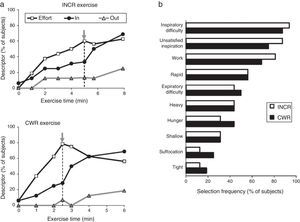
A patient's physical activity is characterised by a growing mismatch between an increase in central neural command to the ventilatory musculature and the blunted respiratory mechanical and/or muscular response (lung/chest wall displacement). It has been suggested that this mismatch, termed neuroventilatory dissociation, is part of the neurophysiological basis of the feeling of unsatisfied inspiration. In a clinical setting, the slope that defines neuroventilatory dissociation (i.e., effort versus displacement) is steeper and shifted upward in patients compared with healthy subjects. At any respiratory workload, a steeper slope will therefore result in a greater intensity of dyspnoea. In particular, patients experience intolerable dyspnoea during exercise because VT expansion is constrained (by the effects of dynamic lung hyperinflation or the already critically reduced resting inspiratory capacity23). As mentioned above, this so-called dyspnoea threshold seems to lie at the level at which the inspiratory reserve volume (IRV) critically approaches 0.5L.22 Once this critical IRV is achieved, further expansion in VT is greatly impaired, the effort-volume displacement ratio (Poes/PI,max: VT/VC) increases sharply and dyspnoea intensity rises steeply to intolerable levels,19–22 regardless of the exercise testing protocol (incremental or constant work rate).19 In addition, the attainment of critical constraints on VT expansion also marks the point of transition in the dominant qualitative descriptor choice from “work and effort” to “unsatisfied inspiration”19 (Fig. 3). Intensity and quality of dyspnoea evolve separately and are strongly influenced by mechanical constraints on VT expansion during exercise in COPD.19,20 These data support the importance of mechanical restriction in causing dyspnoea in COPD patients.24
(A) Choice of dyspnoea descriptors evaluated during incremental (INCR) and constant work rate (CWR) exercise: increased work and effort (Effort), unsatisfied inspiration (IN), and unsatisfied expiration (OUT). The grey arrow represents the inflection point between tidal volume and minute-ventilation. (B) Choice of dyspnoea descriptor evaluated at the end of the exercise test. Adapted from Laveneziana P et al. American Journal of Respiratory and Critical Care Medicine 2011;184:1367–1373, with permission.
Patients with NMD have increased neuromotor output, which is interpreted as increased ventilatory muscle work and as such is thought to be one of the main determinants of dyspnoea in NMD.25 Nonetheless, a significant positive relationship between increased dyspnoea per unit increase in ventilation and dynamic elastance affects the coupling between respiratory effort and displacement.25
Interstitial Lung Disease (ILD)As in COPD, restrictive dynamic respiratory mechanics limit the ability of patients with ILD to increase ventilation in response to increased metabolic demands associated with physical tasks.26 One of the characteristic features of ILD is a reduction in lung compliance and lung volumes. This has two major consequences: (1) greater motor output and work are required from the inspiratory muscles; (2) the resting TLC and IRV are often diminished compared with healthy individuals. Therefore, the increase in VT required during exercise is limited in the early stages “from above” (reflecting the reduced TLC and IRV), which will force a rapid, shallow and less effective pattern of breathing. Differences in dynamic ventilatory mechanics, including possible expiratory flow limitation in some patients, lead to distinct qualitative perception in ILD patients, namely inspiratory difficulty/unsatisfied inspiration and rapid shallow breathing.18,26 Because of the increase in both dynamic elastance and efferent respiratory drive, inspiratory difficulty/unsatisfied inspiration may originate from awareness of uncoupling between the increased ventilatory neural drive (and increased respiratory effort, i.e., Poes/PI,max) and the restricted mechanical response of the respiratory system (i.e., VT/VC), in other words, the impossibility of expanding VT proportionally to the increasing respiratory drive.26 It has also been suggested that the intensity of exertional dyspnoea in ILD is more closely linked to mechanical constraints on volume expansion than to indexes of inspiratory effort per se.26
Chronic Heart Failure (HF)The key message that has emerged from therapeutic intervention studies in patients with HF is that exertional dyspnoea alleviation is consistently associated with reduced excessive ventilatory demand (secondary to reduced central neural drive), improved respiratory mechanics and muscle function and, consequently, enhanced neuromechanical coupling of the respiratory system during exercise.18 Pressure support has been reported to reduce the tidal inspiratory pleural pressure-time slope without affecting submaximal dyspnoea ratings, but allowed patients to exercise for additional minutes without experiencing any significant rise in dyspnoea.27 Laveneziana and collaborators showed that biventricular pacing was associated with improved dyspnoea intensity at a given VE and oxygen consumption (V′O2). The dyspnoea/VE slopes were consistently reduced by ∼50% during exercise in response to active cardiac pacing.28 Improved dyspnoea during active pacing (compared with inactive pacing modality) was associated with (1) reduced ventilatory requirement likely due to (a) delayed onset of metabolic acidosis secondary to improved oxygen delivery or utilisation or both (as suggested by a consistent increase in the anaerobic threshold), and (b) improved ventilation-perfusion relationship as a result of an improved ability to reduce a higher physiological dead-space during exercise due to improved pulmonary perfusion (as suggested by the improved VE/VCO2 slope and ratios), (2) improved dynamic operating lung volumes [due to either reduced dynamic hyperinflation (as reflected by the increased inspiratory capacity on exertion), improved respiratory muscle function, or both], and an increased ability to expand VT during exercise.28 The available data suggest that increased ventilatory demand, abnormal dynamic ventilatory mechanics and respiratory muscle dysfunction are instrumental in causing exertional dyspnoea in patients with severe cardiac impairment.27,28
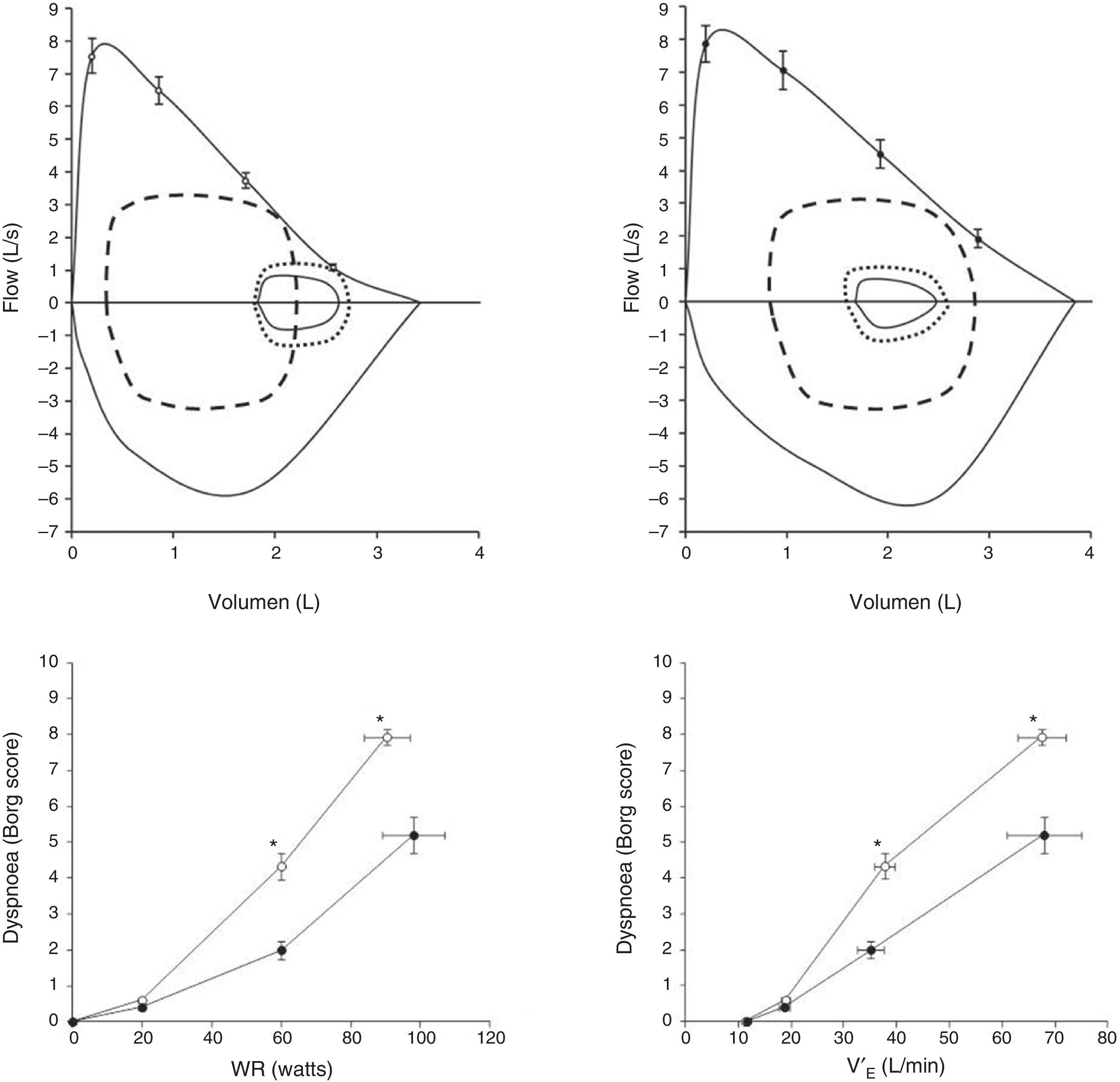
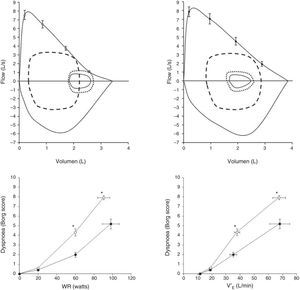
Pulmonary Arterial Hypertension (PAH)Exertional dyspnoea is by far the dominant symptom in patients with pulmonary arterial hypertension (PAH).29–31 Recent advances in PAH have clearly demonstrated that some PAH patients (up to 60%) may exhibit reduced expiratory flows in tidal operating range, which could promote exercise-induced dynamic lung hyperinflation (Fig. 4).29 Laveneziana and collaborators described the impact of potential dynamic lung hyperinflation-induced on the intensity of dyspnoea during CPET in young non-smoking patients with idiopathic and hereditary PAH.29 They showed that reduced expiratory flows at low lung volumes are found in as much as 60% of PAH patients, despite preservation of the FEV1/VC ratio. When increased ventilation/perfusion mismatching was superimposed on pre-existing abnormal airway function, exertional symptoms worsened. Although dyspnoea in these patients was likely multifactorial, the results of the study clearly indicate that increased ventilatory demand and abnormal dynamic ventilatory mechanics should be considered in PAH patients,29 in the absence of respiratory muscle dysfunction31 or TLC changes.29
Upper panels: maximal and tidal flow-volume loops (continuous lines) in patients with pulmonary arterial hypertension (PAH) with exercise-induced hyperinflation (PAH-H; n=15, age 40±11yr; upper left panel) and without hyperinflation (PAH-NH; n=10, age 35±13yr; upper right panel). Flow-volume loops are shown for early exercise (small dotted line) and at peak exercise (dashed line). In the left panel, a decrease in inspiratory capacity is observable, confirming dynamic hyperinflation.
Lower panels: Dyspnoea on exertion (rated using the Borg scale) with increasing work rate (left panel), (WR; lower left panel) and minute-ventilation (V′E; lower right panel) during incremental cycle exercise in patients with PAH-H (open circles) and in PAH-NH patients (filled circles). Data show values at rest, 20W, 60W and peak exercise. *P<.05, PAH-H versus PAH-NH. Adapted and modified from Laveneziana P et al. The European Respiratory Journal. March 2013;41(3):578–587, with permission.
An increase in respiratory neural drive is thought to be the reason for the similar increase in dyspnoea in obese and lean subjects.32 However, different underlying mechanisms may affect dyspnoea in obese subjects. Exercise performance is impaired compared with healthy normal-weight subjects when corrected for the increased lean body mass,33 but is normal when expressed as a percentage of predicted for ideal body weight in subjects who hyperinflate to the same extent as obese subjects who “deflate”, with both groups reaching similar dyspnoea scores.34 In “hyperinflators”, dynamic hyperinflation along with a decrease in inspiratory reserve volume increases respiratory muscle loading, respiratory drive and perception of respiratory discomfort.34 In contrast, “deflators” exhibit a negative relationship between resting end-expiratory lung volume (EELV) and perceptual respiratory response during exercise: the lower the EELV, the greater the Borg score.34 A low resting EELV has three important and related consequences during exercise: (1) a decrease in expiratory reserve volume, (2) dynamic airway compression, and (3) changes in transmural airway pressure resulting in dynamic airway compression. Thus, an alteration in the central drive to the respiratory muscles in response to afferent activity from upper airway mechanoreceptors may also contribute to the unpleasant respiratory sensation in obese subjects.
AsthmaThree main qualitative dyspnoea descriptors are usually seen in asthmatic patients and have been described in conditions ranging from induced bronchoconstriction to physical exercise: (1) chest tightness, (2) increased work/effort (“breathing requires more work or effort”), and (3) unsatisfied/unrewarded inspiration (“can’t get enough air in”).35–37 The relationship between the sensory and mechanical factors have been described in the context of a methacholine challenge test,37–39 allowing the intensity36,40,41 and quality36,38,42 of dyspnoea to be evaluated over a spectrum of bronchoconstrictive responses, from minor bronchoconstriction to severe lung hyperinflation.36,37 Mechanical elements, such as the hyperinflation-induced loading of the respiratory muscles,37,43 are thought to influence the perception of dyspnoea during acute bronchoconstriction37,44 and exercise35 in patients with asthma. Dyspnoea descriptors have also been shown to be a useful addition to standard severity ratings of dyspnoea intensity when monitoring response to therapy with bronchodilators in acute bronchospasm.45
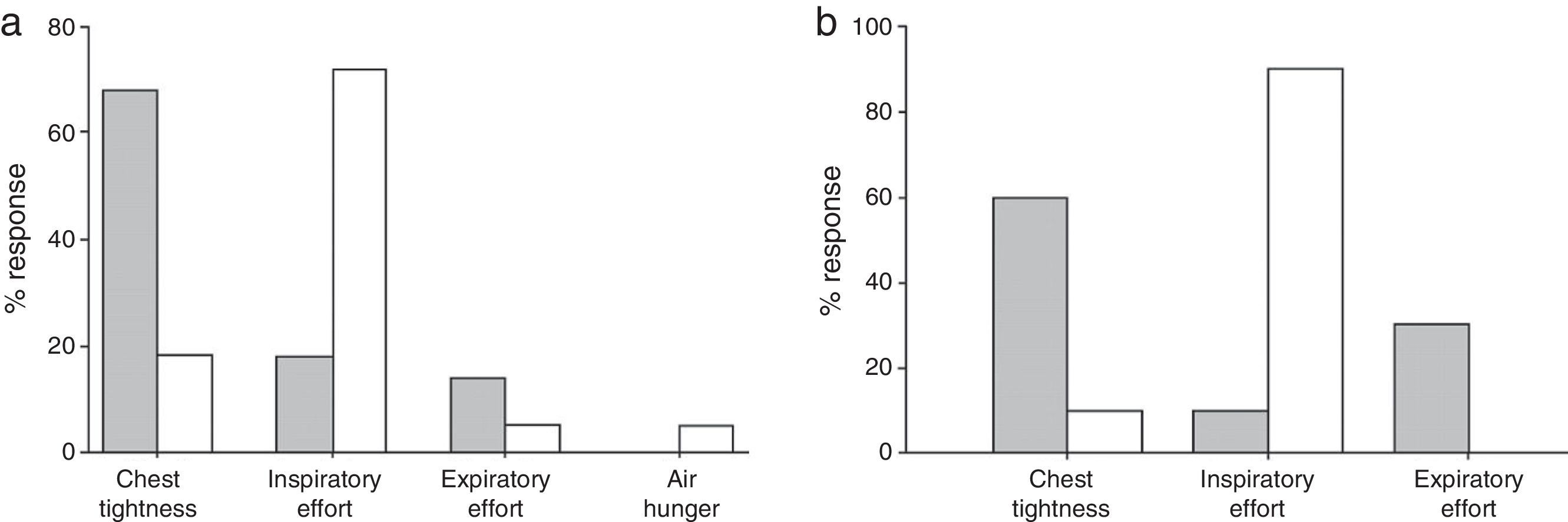
Bronchial asthma is associated with a variety of dyspnoea descriptors, which suggests the existence of numerous mechanisms underlying dyspnoea in this population. This spectrum of dyspnoea descriptors could distinguish between the various causes of dyspnoea in this population.42 Aware that airway obstruction and dynamic hyperinflation have different effects on the language of dyspnoea in the setting of a bronchial hyperreactivity test,37,38 and that the increase in operating lung volumes during short exercise sessions do not seem to be an important contributor to exercise limitation and the intensity of dyspnoea in asthma,46 Laveneziana and collaborators36 hypothesised that various descriptors would be chosen by asthmatic patients during short exercise sessions and methacholine challenge, irrespective of the associated severity of dyspnoea (Fig. 5A). They found that airway obstruction and dynamic lung hyperinflation correlated best with the intensity of dyspnoea during the challenge test.36Chest tightness was the most frequently reported descriptor during the test.36 During a short cycling session, VE was the descriptor best related to dyspnoea, although work/effort was the most frequently reported descriptor.36 The inspiratory capacity decreased in eight subjects during exercise. Importantly, in those subjects the magnitude of dynamic hyperinflation was similar to that observed during the methacholine test, but their description of subjective dyspnoea differed between the two tests (Fig. 5B).36
Choice of dyspnoea descriptor after methacholine challenge (grey columns) and cardiopulmonary exercise test (white columns) (panel A). Panel B shows the same information, limited to subjects with a similar degree of hyperinflation at the end of the tests. Adapted from Laveneziana P et al. The European Respiratory Journal. 2006;27:742–747, with permission.
More recently, Laveneziana and collaborators35 have suggested that expiratory flow limitation at rest and during exercise may be directly related to the extent of the fall in inspiratory capacity (i.e., dynamic lung hyperinflation) observed in patients with stable mild asthma performing a constant work-rate cycle test at high-intensity. Indeed, they found a strong correlation between the extent of expiratory flow limitation and the reduction in inspiratory capacity during exercise.35 However, the lack of oesophageal pressure-derived measurements prevented the evaluation of the contribution of other potential factors influencing the development of dynamic lung hyperinflation.
In this study, the qualitative description of dyspnoea was also evaluated.35 The results showed that approximately one third of subjects reached a point of tidal volume expansion constraint induced by dynamic hyperinflation, resulting in a sharp rise in dyspnoea, whereas the rest did not. The critical point in tidal volume expansion was associated with a sharp rise in dyspnoea and a change in the dominant descriptor or dyspnoea from work/effort to difficult/unsatisfied inspiration35 By contrast, the remaining subjects showed no limitation in VT expansion, and did not change their choice of descriptor, suggesting that the onset of tidal volume expansion limitation is a critical mechanical turning point that has significant sensory consequences.35 From a clinical perspective, these data suggest that even in mild asthma therapeutic interventions that delay the onset of VT constraints at a critically reduced IRV should reduce dyspnoea during exercise.
Effects of Interventions on DyspnoeaImprovement of exertional dyspnoea represents one of the most important factors in the successful management of patients with cardiac and pulmonary diseases. Therapeutic strategies aimed at improving exertional dyspnoea in these conditions usually revolve around interventions that: (1) try to decrease ventilatory demands and/or reduce respiratory drive; (2) increase respiratory capacity; (3) improve exertional respiratory mechanics (by reducing the mechanical load); (4) increase the pressure-generating capacity of the respiratory muscles, and (5) alter the emotional component of dyspnoea. The underlying specific pathophysiological mechanisms responsible for the development of dyspnoea in each subject should guide the choice of a therapeutic intervention specific to the subject being evaluated. However, concomitant interventions are generally required, and these are likely to have synergistic effects.
Among the frequently used therapeutic options that have been shown to be effective in the management of exertional dyspnoea are: bronchodilators,47,48 long-term use of exertional oxygentherapy,49 heliox,50 exercise training,51–53 biventricular pacing (specific for HF patients),28 respiratory muscle training,54 biofeedback techniques,55 non-invasive ventilation,56 lung volume reduction surgery57 and related endoscopic techniques. In a selected subset of patients, other interventions such as opioids have been showed to reduce respiratory drive and positively alter the emotional components of dyspnoea.58 In addition, inhaled furosemide may modulate breathing sensation by modifying afferent inputs from vagal receptors within the lungs.59 Psychological counselling and anxiolytics60,61 can also have a positive influence on the affective dimension of chronic dyspnoea.
ConclusionsDyspnoea is a multifaceted experience that stems from the interaction between physiological, psychological and environmental factors, and can only be understood using a multidisciplinary and multidimensional approach. Although mechanical factors are important contributors to dyspnoea, the exact mechanisms involved remain obscure. One approach to the study of this symptom is to determine the major qualitative dimensions of the symptom in an attempt to identify different underlying neurophysiological mechanisms. The observation that patients with obstructive or restrictive respiratory diseases display relatively narrow and overlapping spectrums of qualitative dyspnoea descriptors (work/effort, inspiratory difficulty/unsatisfied inspiration, air hunger, rapid breathing) raises the question of whether they may share some common underlying mechanisms.
Conflicts of InterestThe authors declare no conflicts of interest
Please cite this article as: Dubé B-P, Vermeulen F, Laveneziana P. Disnea de esfuerzo en las enfermedades respiratorias crónicas: de la fisiología a la aplicación clínica. Arch Bronconeumol. 2017;53:62–70.