The clinical benefits of adjunctive corticosteroids for Pneumocystis jirovecii pneumonia in patients not infected with the human immunodeficiency virus (HIV) has not been evaluated by meta-analysis.
MethodsWe conducted a systematic review of published studies describing the effects of adjunctive corticosteroids on outcome in non-HIV P. jirovecii pneumonia patients. Two investigators independently searched the PubMed and Cochrane databases for eligible articles written in English. A meta-analysis was performed using a random-effects model for measuring mortality as the primary outcome, and the need for intubation or mechanical ventilation as the secondary outcome.
ResultsSeven observational studies were eligible. In these studies, adjunctive corticosteroids did not affect mortality in non-HIV patients (odds ratio [OR] 1.26; 95% CI 0.60–2.67) and there was no beneficial effect in patients with severe hypoxemia (PaO2 <70mmHg) (OR 0.90; 95% CI 0.44–1.83). No significant effect on the secondary outcome was observed (OR 1.34; 95% CI 0.44–4.11).
ConclusionsAlthough the studies were observational, meta-analysis showed that adjunctive corticosteroids did not improve the outcome of P. jirovecii pneumonia in non-HIV patients. The results warrant a randomized controlled trial.
Los beneficios clínicos del tratamiento complementario con corticoides de la neumonía por Pneumocystis jirovecii (P. jirovecii) en pacientes no infectados por el virus de la inmunodeficiencia humana (VIH) no se han evaluado mediante metanálisis.
MétodosRealizamos una revisión sistemática de los estudios publicados que describen los efectos del tratamiento complementario con corticoides sobre la evolución de pacientes con neumonía por P. jirovecii no infectados por VIH. Dos investigadores hicieron búsquedas independientes de artículos elegibles escritos en inglés en las bases de datos PubMed y Cochrane. Se efectuó un metanálisis con un modelo de efectos aleatorios para determinar la mortalidad como variable principal, y la necesidad de intubación o ventilación mecánica como variable secundaria.
ResultadosSiete estudios observacionales resultaron elegibles. En ellos, el tratamiento complementario con corticoides no afectó a la mortalidad en los pacientes no infectados por VIH (odss ratio [OR] 1,26; IC 95% 0,60–2,67) y no tuvo ningún efecto beneficioso para los pacientes con hipoxemia intensa (PaO2<70mmHg) (OR 0,90; IC 95% 0,44–1,83). No se observó ningún efecto significativo sobre la variable secundaria (OR 1,34; IC 95% 0,44–4,11).
ConclusionesAunque los estudios eran observacionales, el metanálisis mostró que el tratamiento complementario con corticoides no mejoraba la evolución de los pacientes con neumonía por P. jirovecii no infectados por VIH. Estos resultados justifican la realización de un ensayo controlado aleatorizado.
Pneumocystis jirovecii pneumonia (PJP) is life-threatening opportunistic infection affecting immunocompromised individuals.1 CD4+ T-cells play crucial roles in host defense against P. jirovecii in response to antigens and the production of interferon-gamma.2 CD4+ T-cells are especially suppressed in cases of advanced human immunodeficiency virus (HIV) infection; however, other causes of immunosuppression are frequently observed in clinical settings, such as continuous corticosteroid use, hematological malignancies, solid organ tumors, and organ transplants.3,4 PJP is most common in patients with HIV; however, PJP in non-HIV patients should not be overlooked, particularly in immunocompromised patients.
The epidemiology and clinical presentations differ between HIV and non-HIV patients. PJP in non-HIV patients occurs at older age, with higher neutrophil levels and a lower density of P. jirovecii in bronchoalveolar lavage compared with HIV patients.5 Furthermore, the onset of respiratory failure is abrupt in non-HIV patients, whereas it is slower in HIV patients.1 Overall, the outcome of PJP is less favorable in non-HIV than HIV-infected patients due to a variety of underlying medical conditions.2
The therapeutic strategy for PJP has been widely studied, especially in HIV-infected individuals. Sulfamethoxazole-trimethoprim is generally adminstered,6 and HIV-PJP patients with substantial hypoxemia are prescribed concurrent adjunctive corticosteroids, based on randomized controlled trials from 1990.7 Recently, a meta-analysis and systematic review showed that adjunctive corticosteroid treatment had a beneficial effect on mortality in patients with hypoxemia (arterial oxygen partial pressure >70mmHg or an alveolar-arterial gradient <35mmHg on room air).8
The value of adjunctive corticosteroid in non-HIV-PJP patients, however, remains unclear. Although several observational studies have been published, they do not show a definitive effect on outcome. There have been no randomized controlled studies conducted to date; this might due to variations in underlying diseases and pathophysiology, and the relative rarity of non-HIV-PJP cases. However, since the pathophysiology of non-HIV-PJP patients differs from HIV-infected PJP patients, there may be factors associated with the use of adjunctive corticosteroids that affect the clinical course.
We conducted a systematic review and meta-analysis of published observational studies focusing on adjunctive corticosteroids in non-HIV-PJP patients.
MethodsStudy SearchA literature search was performed by 2 investigators (YF and TM) who independently searched PubMed and the Cochrane Database of Systematic Reviews for eligible articles published from 1949 to June 2015. We used free search terms, MeSH terms, and combinations of search terms “Pneumocystis”, “pneumonia, Pneumocystis [MeSH]”, “Pneumocystis infections [MeSH]”, “Pneumocystis carinii [MeSH]”, “Pneumocystis jirovecii [MeSH]”, “PCP”, “PJP”, AND “non-HIV”, “non-AIDS”, “non-HIV-infected”, “HIV-uninfected”, “AIDS uninfected”, “HIV-negative”, AND “steroids”, “corticosteroids”, “hydroxycorticosteroids”, “glucocorticoids”, “prednisolone”, “hydroxycortisone”, and “hydrocortisone”, with language restricted to English.
Eligibility Criteria and Outcome MeasuresStudies that met the eligibility criteria were included in the meta-analysis. (1) Study design: randomized controlled trials (RCT) and observational studies; (2) population: non-HIV patients with PJP; (3) intervention: administration of adjunctive corticosteroids; (4) comparison intervention: no adjunctive corticosteroids or no increase in corticosteroid use, according to each study definition; (5) outcome variables: mortality as primary endpoint, and intubation rate (or need for mechanical ventilation) as the secondary endpoint. In addition, for the studies in patients with hypoxemia, we also focused on mortality due to severe hypoxemia (PaO2 <70mmHg) in non-HIV cases of PJP. We excluded articles that did not define HIV status in the study population or did not extract exact data for evaluation.
Statistical AnalysisMeta-analysis was performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis)9 and MOOSE (Meta-analysis Of Observational Studies in Epidemiology) recommendations.10 Data were analyzed using Review Manager (RevMan) 5.3.5 (Cochrane Collaboration, Copenhagen) and R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria) software with an open source statistical package for meta-analysis. A random effect model was used to aggregate data, and the odds ratio (OR) was used in summary. The heterogeneity of original studies was evaluated with I2 statistics. Significance levels were two-tailed in all analyses, and P<.05 was considered significant. A funnel plot examined publication bias.
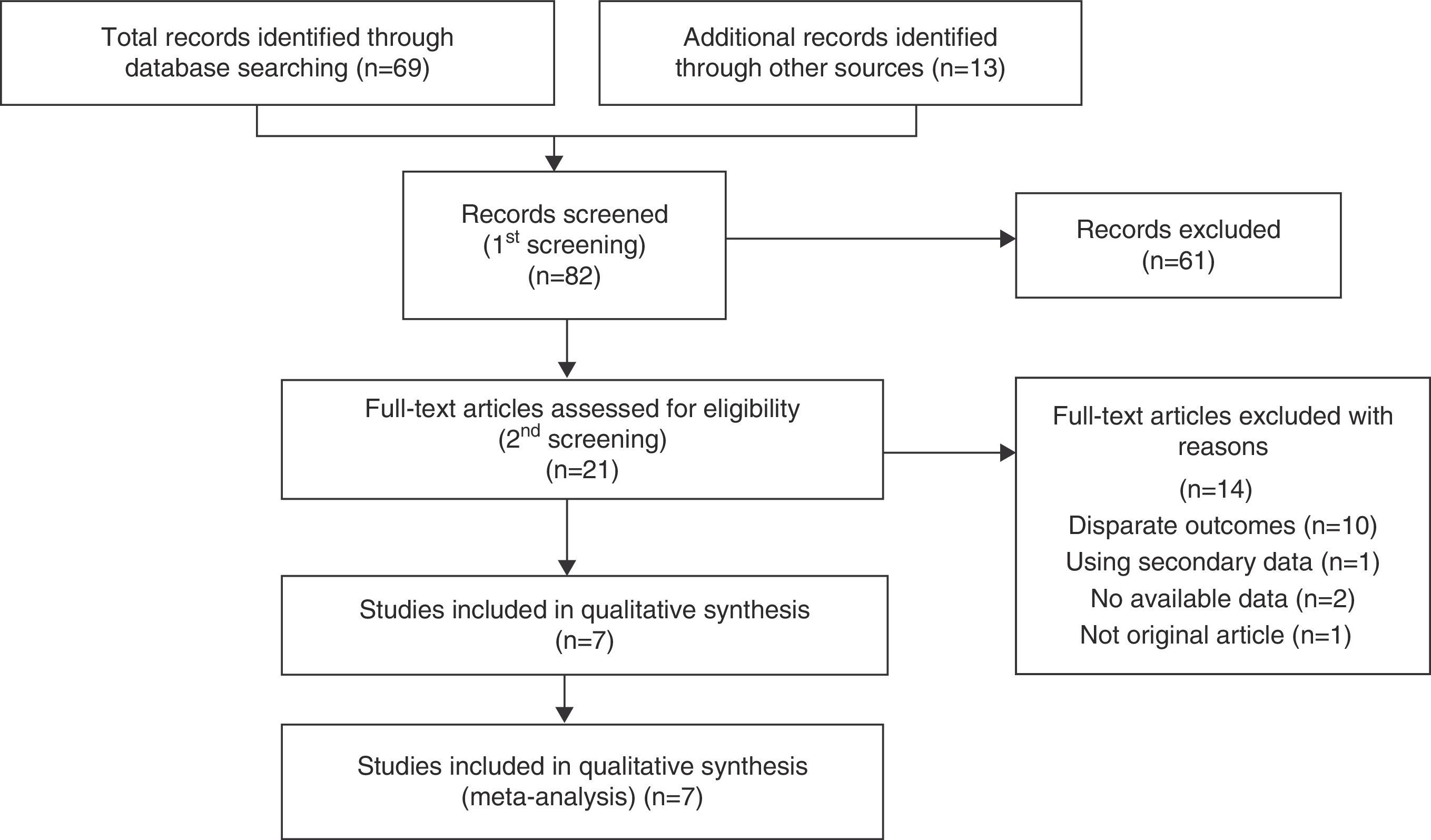
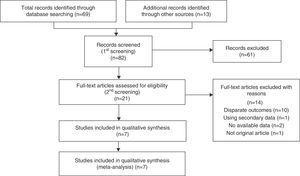
ResultsSearch Results and Study CharacteristicsFollowing discussions between the 2 reviewers, 7 observational studies11–17 were collected by systematic searches according to the PRISMA statement (Fig. 1). The reviewers were in complete agreement about the search performed and data extracted. Of the 82 studies screened, 7 were included in a qualitative synthesis.
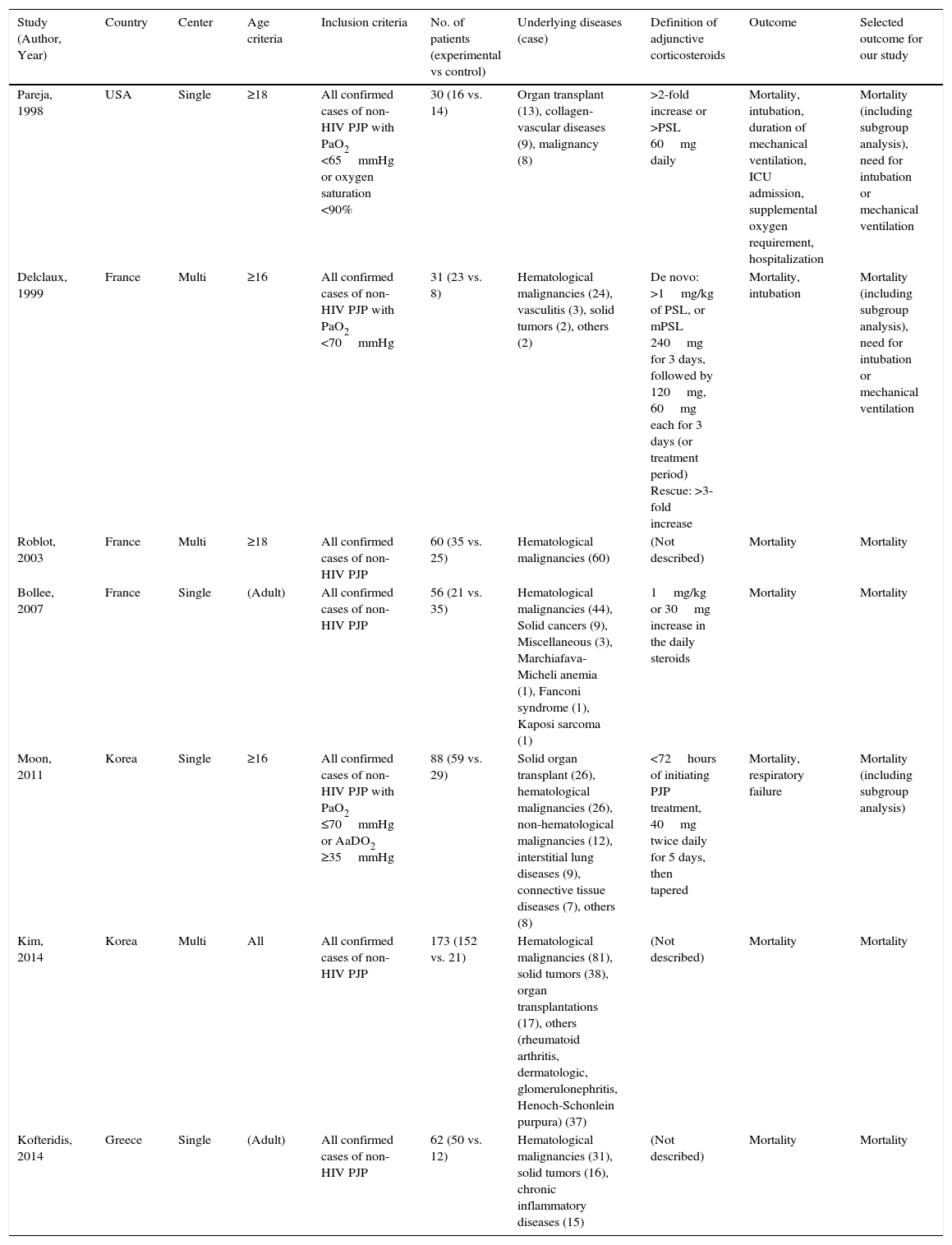
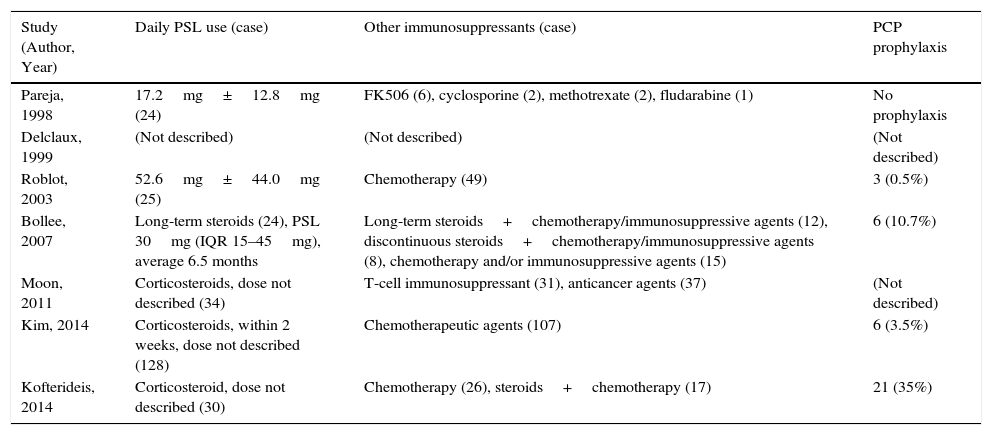
Table 1 shows the characteristics of the studies included. There were no RCTs, and all studies were retrospective observational. The underlying diseases leading to immunosuppression varied widely, but malignancies – especially hematological diseases – were often recorded. Other background patient information is described in Table 2. Corticosteroids were used in 38.6%–80% of patients, and other immunosuppressants were commonly administrated at the time of PJP diagnosis. Notably, prophylaxis against P. jirovecii was less common in non-HIV patients.
Characteristics of Observational Studies Included.
| Study (Author, Year) | Country | Center | Age criteria | Inclusion criteria | No. of patients (experimental vs control) | Underlying diseases (case) | Definition of adjunctive corticosteroids | Outcome | Selected outcome for our study |
|---|---|---|---|---|---|---|---|---|---|
| Pareja, 1998 | USA | Single | ≥18 | All confirmed cases of non-HIV PJP with PaO2 <65mmHg or oxygen saturation <90% | 30 (16 vs. 14) | Organ transplant (13), collagen-vascular diseases (9), malignancy (8) | >2-fold increase or >PSL 60mg daily | Mortality, intubation, duration of mechanical ventilation, ICU admission, supplemental oxygen requirement, hospitalization | Mortality (including subgroup analysis), need for intubation or mechanical ventilation |
| Delclaux, 1999 | France | Multi | ≥16 | All confirmed cases of non-HIV PJP with PaO2 <70mmHg | 31 (23 vs. 8) | Hematological malignancies (24), vasculitis (3), solid tumors (2), others (2) | De novo: >1mg/kg of PSL, or mPSL 240mg for 3 days, followed by 120mg, 60mg each for 3 days (or treatment period) Rescue: >3-fold increase | Mortality, intubation | Mortality (including subgroup analysis), need for intubation or mechanical ventilation |
| Roblot, 2003 | France | Multi | ≥18 | All confirmed cases of non-HIV PJP | 60 (35 vs. 25) | Hematological malignancies (60) | (Not described) | Mortality | Mortality |
| Bollee, 2007 | France | Single | (Adult) | All confirmed cases of non-HIV PJP | 56 (21 vs. 35) | Hematological malignancies (44), Solid cancers (9), Miscellaneous (3), Marchiafava-Micheli anemia (1), Fanconi syndrome (1), Kaposi sarcoma (1) | 1mg/kg or 30mg increase in the daily steroids | Mortality | Mortality |
| Moon, 2011 | Korea | Single | ≥16 | All confirmed cases of non-HIV PJP with PaO2 ≤70mmHg or AaDO2 ≥35mmHg | 88 (59 vs. 29) | Solid organ transplant (26), hematological malignancies (26), non-hematological malignancies (12), interstitial lung diseases (9), connective tissue diseases (7), others (8) | <72hours of initiating PJP treatment, 40mg twice daily for 5 days, then tapered | Mortality, respiratory failure | Mortality (including subgroup analysis) |
| Kim, 2014 | Korea | Multi | All | All confirmed cases of non-HIV PJP | 173 (152 vs. 21) | Hematological malignancies (81), solid tumors (38), organ transplantations (17), others (rheumatoid arthritis, dermatologic, glomerulonephritis, Henoch-Schonlein purpura) (37) | (Not described) | Mortality | Mortality |
| Kofteridis, 2014 | Greece | Single | (Adult) | All confirmed cases of non-HIV PJP | 62 (50 vs. 12) | Hematological malignancies (31), solid tumors (16), chronic inflammatory diseases (15) | (Not described) | Mortality | Mortality |
PSL, prednisolone; mPSL, methyl-prednisolone; PJP, Pneumocystis jirovecii pneumonia.
Treatment Profiles of Patients in Studies.
| Study (Author, Year) | Daily PSL use (case) | Other immunosuppressants (case) | PCP prophylaxis |
|---|---|---|---|
| Pareja, 1998 | 17.2mg±12.8mg (24) | FK506 (6), cyclosporine (2), methotrexate (2), fludarabine (1) | No prophylaxis |
| Delclaux, 1999 | (Not described) | (Not described) | (Not described) |
| Roblot, 2003 | 52.6mg±44.0mg (25) | Chemotherapy (49) | 3 (0.5%) |
| Bollee, 2007 | Long-term steroids (24), PSL 30mg (IQR 15–45mg), average 6.5 months | Long-term steroids+chemotherapy/immunosuppressive agents (12), discontinuous steroids+chemotherapy/immunosuppressive agents (8), chemotherapy and/or immunosuppressive agents (15) | 6 (10.7%) |
| Moon, 2011 | Corticosteroids, dose not described (34) | T-cell immunosuppressant (31), anticancer agents (37) | (Not described) |
| Kim, 2014 | Corticosteroids, within 2 weeks, dose not described (128) | Chemotherapeutic agents (107) | 6 (3.5%) |
| Kofterideis, 2014 | Corticosteroid, dose not described (30) | Chemotherapy (26), steroids+chemotherapy (17) | 21 (35%) |
PSL, prednisolone; IQR, inter-quartile range; PCP, Pneumocystis jirovecii pneumonia.
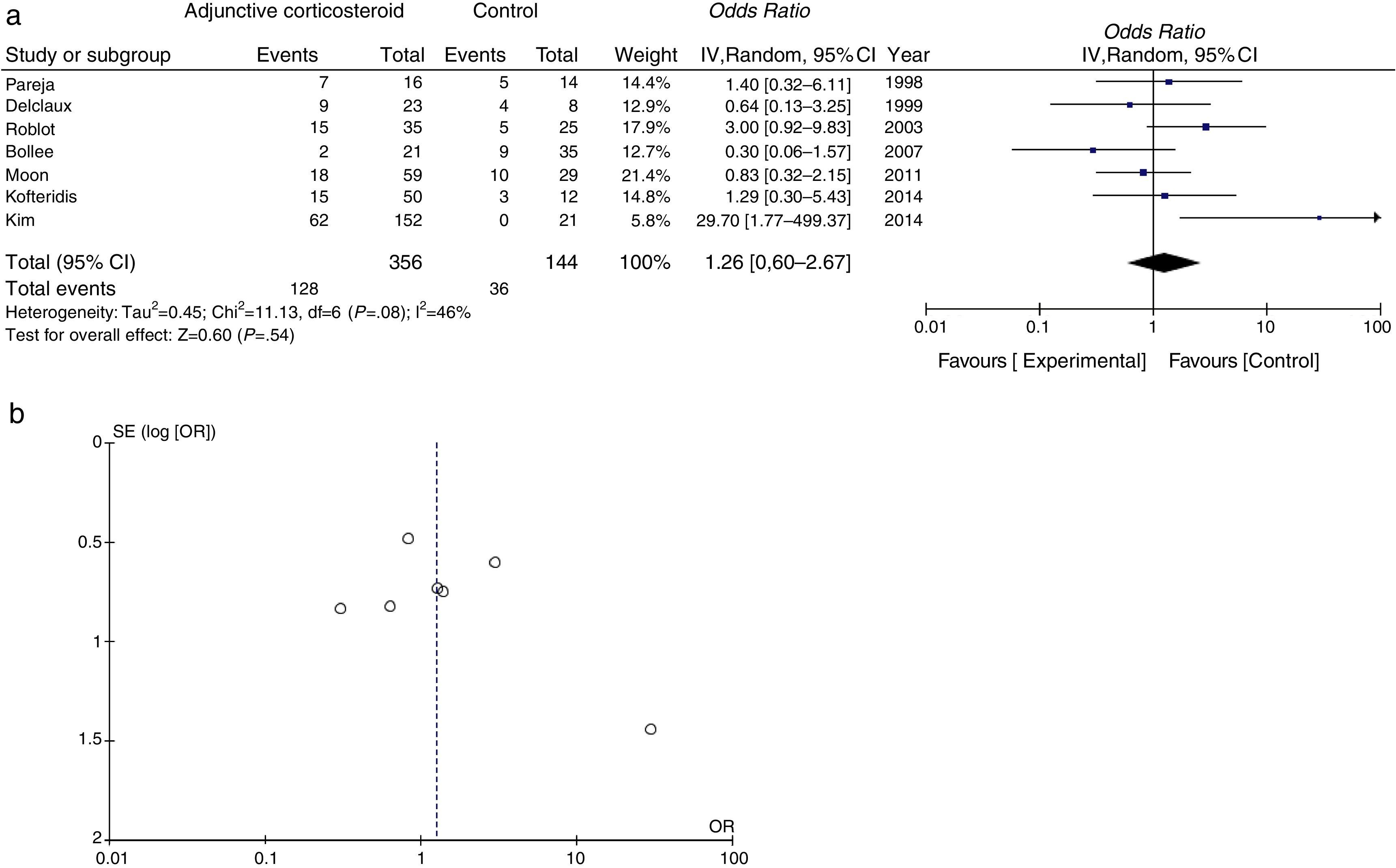
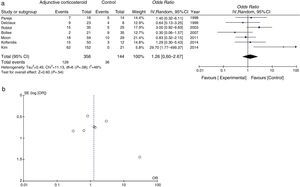
Mortality data were available for all studies. The pooled OR for mortality with adjunctive corticosteroids estimated from the results of the 7 observational studies was 1.26 (95% confidence interval [95% CI], 0.60–2.67, P=.54, I2=46%, P for heterogeneity=.08), which shows no effective outcome for the use of adjunctive corticosteroids in non-HIV patients (Fig. 2a). Some conflicting results were found, and moderate heterogeneity was observed. A funnel plot for the OR for mortality is shown in Fig. 2b. The slight lack of symmetry among studies indicates a potential publication bias, but this did not reach statistical significance (Egger's test, P=.230).
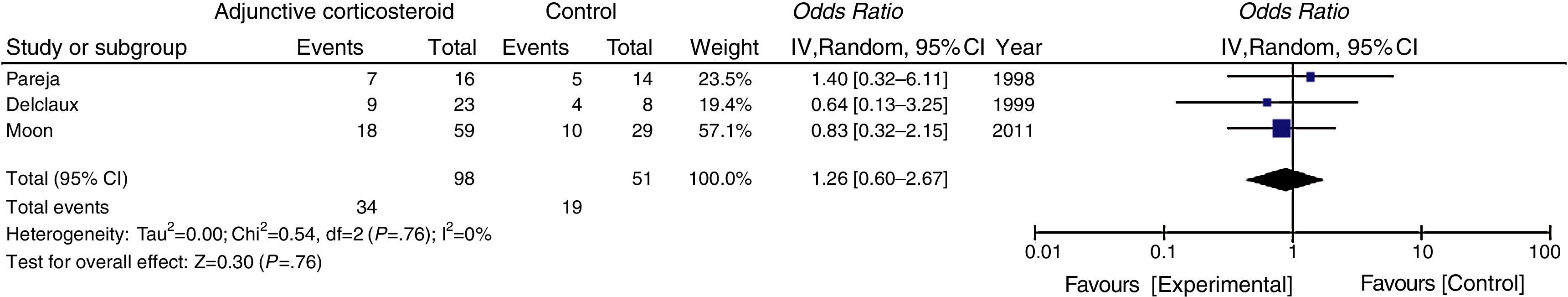
In the subgroup analysis, the mortality of non-HIV-PJP patients with significant hypoxemia (PaO2 <70mmHg) was not reduced via adjunctive corticosteroids (Fig. 3).
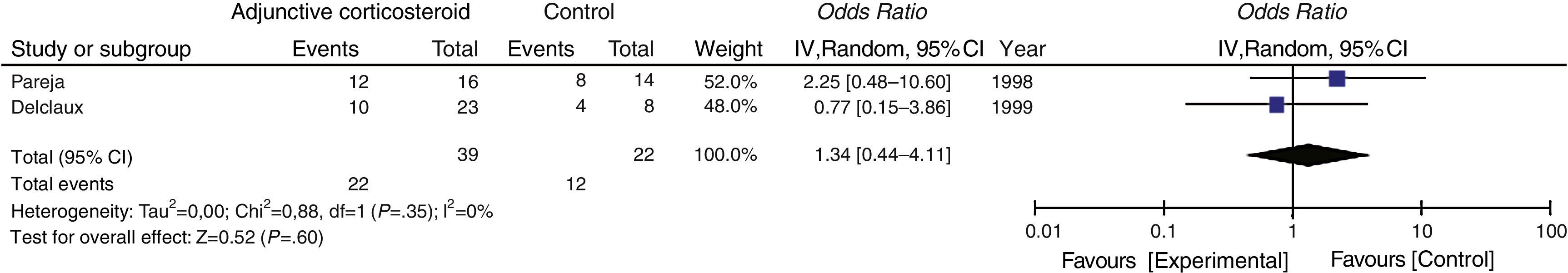
Secondary OutcomeThe need for intubation or mechanical ventilation was evaluated in 2 studies, which yielded a pooled OR of 1.34 (95% CI 0.44–4.11, P=.60, I2=0%, P for heterogeneity=.35) (Fig. 4).
DiscussionIn this meta-analysis, no proof that adjunctive corticosteroids have beneficial effects on mortality in HIV-negative patients with PJP was found, and no statistically significant differences in mortality rate were found between non-HIV-PJP patients with hypoxemia (PaO2 <70mmHg) vs. HIV patients.8 Furthermore, treatment had no effect on the need for intubation or mechanical ventilation, which, according to previous studies, are prognostic factors of clinical course.18,19
Although a randomized clinical trial has showed that administration of corticosteroids significantly reduced the relative risk of death in HIV-infected patients with PJP and substantial hypoxemia,8 all published observational studies for non-HIV-infected patients have failed to show differences in mortality. Moreover, the results of Delclaux,12 Bollee,14 and Moon15 show that adjunctive corticosteroids might have a mildly beneficial effect on mortality, while Kim16 (reporting the largest number of subjects) found that corticosteroids had a negative effect. These results suggest that pathophysiological differences play a role in the disparity between HIV-PJP and non-HIV-PJP patients.
Corticosteroids are concurrently used with anti-pneumocystis treatment in HIV patients with severe hypoxemia and attenuate transient excessive inflammation associated with drug-induced death from the eradication of Pneumocystis jiroveciii,6 which may improve the clinical course. Generally, HIV-infected patients with PJP have a high burden of P. jirovecii in the lung.5 One possible explanation for the lack of effect on mortality among non-HIV-PJP patients might be that the low numbers of microbes in the lung limit the potential benefits.12
Corticosteroid administration, meanwhile, is a major risk factor in the promotion of PJP,20 especially in cases of long-term use in non-HIV patients.3 Prior use of corticosteroids may alter the response to adjunctive corticosteroid treatment, that is, corticosteroids do not have a strong anti-inflammatory effect in PJP patients who have already taken these drugs. Indeed, a previous report revealed that prior corticosteroids use showed a trend toward an increase in mortality among non-HIV patients with PJP.21
Several additional findings from this review are noteworthy. First, the overall mortality rate of PJP in non-HIV patients was 32.8% among all subjects, which exceeds the rate reported in HIV patients with PJP.1 These results might depend on the medical background of the patients rather than the severity of PJP or the pathophysiology of P. jirovecii; however, early diagnosis and treatment are necessary, because delays have been identified as prognostic factors in PJP.22
Second, prophylaxis for P. jirovecii was administered infrequently in all studies. A recent meta-analysis of PJP prevention in non-HIV immunocompromised patients23 indicated an increased need for prophylactic anti-pneumocystis agents. Emphasis should therefore be placed on prophylaxis in patients receiving long-term corticosteroids or other immunosuppressive agents due to the high mortality rate associated with PJP.
Some limitations to this systematic review should be mentioned. First, although publication bias did not reach statistical significance in our meta-analysis, all studies were retrospective and observational in design; thus, the true effects on mortality cannot be ascertained due to the potential for confounding bias. The possibility that adjunctive corticosteroids are administered more frequently in severely ill patients cannot be ruled out, and this might have underestimated the effect of this therapy. However, it might be difficult to conduct a randomized controlled trial, because there are fewer non-HIV-PJP patients and adjunctive corticosteroids are regarded as the standard treatment for PJP by many clinicians regardless of HIV status. From this point of view, our meta-analysis shows a strong trend among non-HIV-PJP patients. Second, the studies available are heterogeneous. Non-HIV-PJP patients constitute a heterogeneous group, which makes stratified subgroup analysis difficult. Statistical significance might not be attainable due to the inconsistent characteristics of non-HIV patients. In addition, the definition of adjunctive corticosteroids was not uniform in the studies included. Insufficient doses of corticosteroids could affect clinical outcome.
ConclusionsAlthough purely observational studies were included, this meta-analysis showed that adjunctive corticosteroids did not improve the outcome of PJP in non-HIV patients. However, this result needs to be confirmed by a randomized controlled trial.
AuthorshipYuji Fujikura: literature search, data collection, analysis of data, manuscript preparation.
Toshie Manabe: literature search, data collection, analysis of data, review of manuscript.
Akihiko Kawana: review of manuscript.
Shigeru Kohno: study design, review of manuscript.
Conflicts of InterestThe authors state that they have no conflict of interests.
This meta-analysis and systematic review was performed while compiling the Japanese Respiratory Society Practical Guidelines for Pneumonia. We would like to thank all members of the Committee.
Please cite this article as: Fujikura Y, Manabe T, Kawana A, Kohno S. Tratamiento complementario con corticoides en la neumonía por Pneumocystis jirovecii en pacientes no infectados por VIH: revisión sistemática y metanálisis de los estudios observacionales. Arch Bronconeumol. 2017;53:55–61.