Fluoroscopy-guided bronchoscopy is usually performed for the diagnosis of peripheral pulmonary lesions (PPL), but the diagnostic yield varies widely among studies. Endobronchial ultrasound (EBUS) can increase the diagnostic yield of bronchoscopic diagnosis of PPL.
ObjectiveTo compare the diagnostic yield of fluoroscopy-guided bronchoscopy and EBUS with fluoroscopy-guided bronchoscopy in the study of PPL.
MethodsAll patients who underwent bronchoscopy to study PPL from January 2009 to December 2012 were prospectively included. A total of 145 consecutive patients were randomly distributed in two groups: EBUS and fluoroscopy (50 patients, 71.3±8.2years) or fluoroscopy alone (95 patients, 68±10.5years). The mean diameter of the lesions was 41.97±19.22mm. Cytological brushing and transbronchial biopsies were obtained. All procedures were performed under fluoroscopic guidance with intravenous conscious sedation. EBUS was performed using an endoscopic ultrasound system equipped with a 20-MHz radial miniprobe introduced via a guide-sheath. Bronchoscopist, cytologist, study protocol, techniques and tools were the same throughout the whole study.
ResultsIn all, 129 (89%) patients had malignant disease. A diagnosis with bronchoscopy was established in 105 (72.4%) patients. EBUS plus fluoroscopy obtained a diagnostic yield in 78% of patients and fluoroscopy alone in 69.5% (non-significant). In contrast, for lesions smaller than 30mm, EBUS plus fluoroscopy guidance provided significantly greater diagnostic performance than fluoroscopy alone (90% vs 52%; P=.05).
ConclusionsBronchoscopy under EBUS plus fluoroscopy guidance is a technique that has become useful for the diagnosis of LPPs, especially those smaller than 30mm in diameter.
La broncoscopia guiada por fluoroscopia se utiliza para el diagnóstico de las lesiones pulmonares periféricas (LPP), pero su rendimiento es muy variable en función de los estudios. La ultrasonografía endobronquial (USEB) radial podría incrementar el rendimiento diagnóstico de la broncoscopia para estas lesiones.
ObjetivosComparar el rendimiento diagnóstico de la broncoscopia guiada por fluoroscopia y USEB radial con la broncoscopia guiada por fluoroscopia para el estudio de LPP.
MétodosSe incluyeron de forma prospectiva todos los pacientes que se sometieron a broncoscopia con fluoroscopia para el estudio de LPP desde enero de 2009 a diciembre de 2012. Los 145pacientes se aleatorizaron en 2grupos: fluoroscopia y USEB radial (50pacientes, 71,3±8,2años) o fluoroscopia únicamente (95pacientes, 68±10,5años). El diámetro medio de las lesiones fue de 41,97±19,22mm. Se tomaron muestras de cepillado bronquial citológico y biopsia transbronquial. Todas las exploraciones se realizaron bajo control fluoroscópico y con sedación intravenosa. Para la USEB se utilizó un procesador ecográfico equipado con una ultra-minisonda ecográfica de 20MHz que se introducía por una guía. Broncoscopista, citólogo, protocolo de estudio, técnicas y utillaje fueron los mismos durante todo el estudio.
ResultadosCiento veintinueve (89%) pacientes presentaban patología maligna. Se obtuvo el diagnóstico por broncoscopia en 105 (72,4%) enfermos. En el grupo con fluoroscopia y USEB radial se diagnosticaron el 78% de los pacientes y en el grupo con solo fluoroscopia el 69,5% (n.s.). Sin embargo, para lesiones menores de 30mm la fluoroscopia con USEB radial incrementaba significativamente el rendimiento diagnóstico comparado con la fluoroscopia únicamente (90 vs 52%; p=0,05).
ConclusionesLa USEB radial asociada a fluoroscopia es una técnica especialmente útil para el diagnóstico de las LPP de un tamaño inferior a 30mm.
Peripheral pulmonary lesions (PPL) are focal radiographic opacities which, depending on their size, are characterized as nodules (≤3cm) or masses (>3cm). The prevalence of malignancy in studies evaluating patients with non-calcified nodules ranges between 2 and 82%, depending on the series.1
The diagnostic yield of conventional bronchoscopy in PPL is usually less than 20%,2,3 and its sensitivity depends on the size of the lesion, its proximity to the bronchial tree and the prevalence of cancer in the studied population.4,5 In the case of relatively large PPLs of 2.5–4cm in diameter, diagnostic efficiency can reach 62%, but it drops below 40% for lesions smaller than 2.5cm.1 Moreover, the diagnostic accuracy of fluoroscopy-guided bronchoscopy varies considerably for this type of injury so, depending on the series, it may vary between 14% and 71%.5–7 Factors potentially limiting the performance of this technique include the location and, in particular, the size of the lesion, and results are reduced to 11%–42% in minor lesions smaller than 2cm.5,7,8
Radial probe endobronchial ultrasound (EBUS) is an ultrasound modality that provides a detailed picture of the bronchial wall and adjacent structures. It consists of an ultrasound miniprobe of 20MHz frequency that, inserted through the working channel of the bronchoscope, provides a 360° ultrasound image. Herth et al.9 were the first to use it in 2002 for PPL studies, and it has proven useful in recent years for increasing the performance of fiberoptic bronchoscopy in the diagnosis of these lesions, especially those smaller than 2–3cm.9–11 In this respect, a recent meta-analysis showed overall diagnostic yield of up to 73%.12
The main objective of this study was to evaluate in our setting the usefulness of radial probe EBUS combined with fluoroscopy-guided bronchoscopy for the diagnosis of patients with PPL, compared to the use of fluoroscopy-guided bronchoscopy alone.
Patients and MethodsThe study was approved by the Ethics Committee of the institution, and the Declaration of Helsinki and local and international regulations for the development of studies in humans were followed at all times. All the patients in whom fluoroscopy-guided bronchoscopy (with or without EBUS) was performed for the diagnosis of peripheral lung injury between January 2009 and December 2012 were prospectively included. Patients were recruited from a dedicated clinical unit that is part of a rapid diagnostic circuit where patients with high suspicion of pulmonary tumor disease are seen. The patients were randomized using standard chest X-ray with the allocation sequence 1:2 (one patient for fluoroscopy with EBUS, two patients for fluoroscopy), which was also subject to the availability of an ultrasound miniprobe during the study period. PPL was considered a radiopacity surrounded by normal lung parenchyma and not visible on endoscope by bronchoscopy. The study was conducted on the basis of multidetector CT scans (Sensation 4 and Sensation 16, Siemens, Ehrlangen, Germany), after the administration of 115ml of intravenous contrast (Ioversol, Optiray 300 Ultraject, TycoHealthcare, Sant Joan Despi, Spain). Size, expressed as major and minor diameters, location and possible existence of a sub-subsegmental bronchus with access to the lesion were specified for each lesion.
ProcedureBronchoscopies were performed in the endoscopy room, equipped with a fluoroscopy unit (Phillips Medical System BV Bracelet; Veenpluis, Netherlands). This equipment consists of a C-arm that provides a two-dimensional image. Briefly, scans were performed after premedication with sublingual diazepam and topical anesthesia with lidocaine 2%, and were carried out under sedation with intravenous propofol and remifentanil, supervised by an anesthesiologist. In all cases, the bronchial tree was examined in both lungs down to subsegmental bronchi and bronchial aspirate (BAS) samples were collected. Olympus (Olympus, BF-180 Q Tokyo, Japan) fiber-optic bronchoscopes were used, with a working channel of 2mm. All procedures were performed by the same bronchoscopist after obtaining informed consent from the patient and normal results in coagulation parameters [platelet >60000/mm3 and prothrombin time (PT)>60%].
Fluoroscopy-guided Bronchial Brushing and Transbronchial BiopsiesBronchial brushes (disposable cytology brush Olympus BC-202D-2010) and transbronchial biopsy forceps (disposable biopsy forceps FB-231D, Olympus) were used. The number of brushings was 2–3. The sample was subsequently smeared on a microscope slide and then immersed in 96° alcohol for subsequent cytological analysis. Samples were stained by the Papanicolaou technique in the pathology laboratory. The number of transbronchial biopsies was 4, and the samples were immersed in formalin for subsequent preparation of a paraffin block.
Fluoroscopy-guided Bronchial Brushing and Transbronchial Biopsies and Radial Probe EBUSBriefly, an ultra-miniprobe UM-S20-17S (Olympus) was inserted through the working channel of the bronchoscope. The probe had a 20MHz ultrasound transducer inside a plastic guide (guide sheath SG-200C, Olympus) (Fig. 1) and was pushed forward in the segmental bronchus until the lesion was located by ultrasound and with the aid of fluoroscopy. Then, the ultra-miniprobe was withdrawn and a specific brush was inserted (disposable cytology brush BC-204D-2010, Olympus) through the plastic guide, followed by a transbronchial biopsy forceps (disposable biopsy forceps FB-233D, Olympus). The number of brushings was 2–3, and the number of transbronchial biopsies, 4.
In both groups, when the results of diagnostic procedures were negative for malignancy, the final diagnosis obtained by other diagnostic techniques (needle aspiration or transthoracic biopsy, both CT-guided) or from surgery was considered as a reference. When the result of these techniques ruled out malignancy and no surgical procedure was indicated, clinical monitoring for at least 6 months was used as reference.
Statistical AnalysisThe data were entered into a database and analyzed using SPSS software v20 (SPSS Inc., Chicago, USA). Descriptive statistics were performed, and expressed as absolute and relative frequencies for categorical variables, and as means and standard deviations (SD) for continuous variables. Frequency data were compared using the χ2 test, and the Student t test was used for comparison of means of independent variables, given the normality of variable distributions. Results were considered significant in case of P<.05. The sensitivity, specificity, and positive and negative predictive values were calculated using standard formulas.
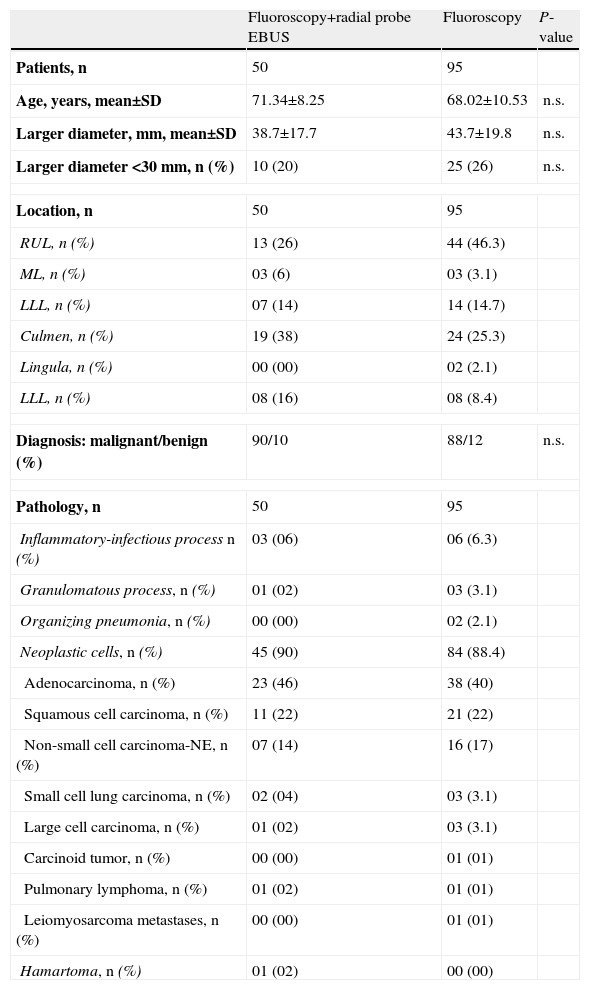
ResultsA total of 145 patients were included, of whom 77.2% were men, and mean age was 69.2±9.9 years. Bronchoscopy was performed with fluoroscopy and radial probe EBUS in 50 cases, and only with fluoroscopy in 95 patients. Mean length of the largest diameter of the lesions was 41.97±19.22mm. Characteristics of the patient groups studied, lesion location and final diagnoses are summarized in Table 1.
Population Characteristics and Final Diagnoses.
| Fluoroscopy+radial probe EBUS | Fluoroscopy | P-value | |
| Patients, n | 50 | 95 | |
| Age, years, mean±SD | 71.34±8.25 | 68.02±10.53 | n.s. |
| Larger diameter, mm, mean±SD | 38.7±17.7 | 43.7±19.8 | n.s. |
| Larger diameter <30mm, n (%) | 10 (20) | 25 (26) | n.s. |
| Location, n | 50 | 95 | |
| RUL, n (%) | 13 (26) | 44 (46.3) | |
| ML, n (%) | 03 (6) | 03 (3.1) | |
| LLL, n (%) | 07 (14) | 14 (14.7) | |
| Culmen, n (%) | 19 (38) | 24 (25.3) | |
| Lingula, n (%) | 00 (00) | 02 (2.1) | |
| LLL, n (%) | 08 (16) | 08 (8.4) | |
| Diagnosis: malignant/benign (%) | 90/10 | 88/12 | n.s. |
| Pathology, n | 50 | 95 | |
| Inflammatory-infectious process n (%) | 03 (06) | 06 (6.3) | |
| Granulomatous process, n (%) | 01 (02) | 03 (3.1) | |
| Organizing pneumonia, n (%) | 00 (00) | 02 (2.1) | |
| Neoplastic cells, n (%) | 45 (90) | 84 (88.4) | |
| Adenocarcinoma, n (%) | 23 (46) | 38 (40) | |
| Squamous cell carcinoma, n (%) | 11 (22) | 21 (22) | |
| Non-small cell carcinoma-NE, n (%) | 07 (14) | 16 (17) | |
| Small cell lung carcinoma, n (%) | 02 (04) | 03 (3.1) | |
| Large cell carcinoma, n (%) | 01 (02) | 03 (3.1) | |
| Carcinoid tumor, n (%) | 00 (00) | 01 (01) | |
| Pulmonary lymphoma, n (%) | 01 (02) | 01 (01) | |
| Leiomyosarcoma metastases, n (%) | 00 (00) | 01 (01) | |
| Hamartoma, n (%) | 01 (02) | 00 (00) | |
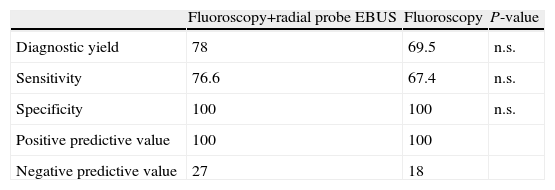
The material obtained by bronchoscopic techniques (BAS/bronchial brushing/transbronchial biopsy) allowed diagnosis in 105 of the 145 patients (72.4%). Final diagnosis was tumor disease in 129 patients (89%), while malignancy was ruled out in the remaining 16 (11%). BAS was positive in 48 patients (33.1%): 16 in the group where fluoroscopy and radial probe EBUS were performed (32%) and only 32 in the fluoroscopy group (33.7%). Only in two cases (1.3%) was BAS the only diagnostic sample. Table 2 shows the diagnostic yield, sensitivity, specificity and predictive values of fiberoptic bronchoscopy in the two groups studied. A tendency toward higher diagnostic yield was observed in the group with fluoroscopy and radial probe EBUS, compared to the group with fluoroscopy alone (78% vs 69.5%), although statistically significant differences were not found.
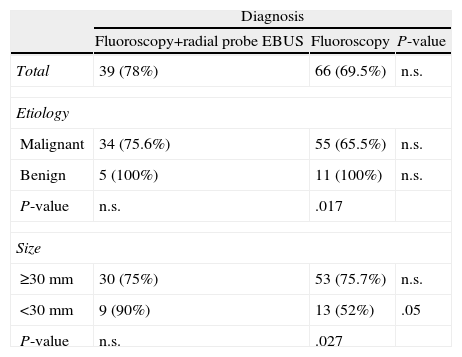
Diagnostic yield according to etiology (benign or malignant) and size (larger or smaller than 30mm) is shown in Table 3. No differences were found between the two groups for lesions equal to or larger than 30mm (75.7% vs 75%, respectively; not significant [n.s.]). In contrast, for lesions smaller than 30mm, fluoroscopy combined with EBUS provided a higher diagnostic yield compared to fluoroscopy alone (52% vs 90%, respectively; P=.05). When the influence of etiology and lesion size on the diagnostic yield within each group was analyzed, differences in the group of fluoroscopy and radial EBUS were not observed, while differences were found when only fluoroscopy was used. Significant differences in yield were not demonstrated for the location in each of the groups studied (fluoroscopy vs fluoroscopy and EBUS): right upper lobe (69% vs 73%, respectively, n.s.), middle lobe (67% vs 33%, respectively), right lower lobe (86% both), culmen (74% vs 58%, respectively) and left lower lobe (87% vs 75%, respectively).
Influence of the Etiology and the Size of the Lesion in Diagnostic Yield.
| Diagnosis | |||
| Fluoroscopy+radial probe EBUS | Fluoroscopy | P-value | |
| Total | 39 (78%) | 66 (69.5%) | n.s. |
| Etiology | |||
| Malignant | 34 (75.6%) | 55 (65.5%) | n.s. |
| Benign | 5 (100%) | 11 (100%) | n.s. |
| P-value | n.s. | .017 | |
| Size | |||
| ≥30mm | 30 (75%) | 53 (75.7%) | n.s. |
| <30mm | 9 (90%) | 13 (52%) | .05 |
| P-value | n.s. | .027 | |
Regarding the bronchoscopic techniques, bronchial brushing was diagnostic in 84 (58%) cases, and transbronchial biopsy in 60 (41.4%), both being positive in 46 patients (31.7%). When analyzed by groups, there were no significant differences when using the ultrasound miniprobe or not, although a trend toward a higher diagnostic yield in both techniques with EBUS was detected (66% vs 53.7% for brushing and 50 vs 36.8% for transbronchial biopsy, respectively).
In the group with fluoroscopy and radial probe EBUS, the lesion was identified by ultrasound in 38 of the 50 patients studied (76%). The diagnostic yield was 95%, versus 25% in other cases (P<.001). In the group with fluoroscopy alone, contact was made with the lesion in 78 of the 95 cases studied (82%). Diagnostic yield when there was contact with the lesion was 78.2% vs 29.4% in patients in whom, despite fluoroscopy, no contact was made with the lesion (P<.001).
A bronchus leading directly to a peripheral pulmonary lesion (bronchus sign) was present in 34 of 47 patients (72.3%) of the fluoroscopy and radial EBUS group, and in 53 of 77 (68.8%) patients with fluoroscopy alone. Its existence could not be determined in other cases. The diagnostic yield in the first group when a bronchus sign was seen was 76.5%, and 84% when not detected (n.s.). In the group examined with fluoroscopy only, no differences were found depending on the presence or absence of bronchus sign (71.7% and 58.3%, respectively, n.s.). Furthermore, in the group with fluoroscopy with EBUS, when the lesion was smaller than 30mm in diameter, bronchus sign was present in 8 of 10 patients in whom its determination was possible (80%), and in 26 of 37 patients (70%) when the lesion measured 30mm or more (n.s.). Presence of bronchus sign, depending on lesion size, was 70% in the group with fluoroscopy and radial probe EBUS, and 78% in the group with fluoroscopy alone (P<.397) in the case of lesions sized greater than or equal to 30mm, and 80% and 44%, respectively (P<.114), for lesions smaller than 30mm.
Insertion of the ultrasound miniprobe in the subsegmental bronchus was not possible in 8 patients (16%) initially included in the fluoroscopy and EBUS group (apical or posterior segments of the upper lobes in all cases), which resulted in bronchial brushing sample collection with fluoroscopic control only.
Procedure time was similar in the two groups: it was 5±2min longer when ultrasound was used but exposure to fluoroscopy was the same.
No major complications were recorded in relation to the performance of radial probe EBUS or intravenous sedation. After transbronchial biopsy, 9 (6%) bleeding episodes were detected that were controlled with standard endoscopic measures. No cases of iatrogenic pneumothorax were detected. In this regard, chest X-ray was performed in all patients 3–4h after bronchoscopy completion.
DiscussionThis study demonstrates the utility of EBUS in increasing the diagnostic yield of fluoroscopy-guided bronchoscopy, especially for smaller lesions measuring less than 30mm.
A recent meta-analysis of Steinfort et al.,12 based on 16 studies involving 1420 patients, determined the sensitivity of EBUS-guided bronchoscopy for the diagnosis of pulmonary tumor disease in 73% (95% CI: 70%–76%), and specificity was 100% (95% CI: 99%–100%). Sensitivity was influenced by the prevalence of malignancy in the population studied. In this regard, when prevalence was lower than 75%, sensitivity was 70% (95% CI: 66%–74%), and 83% (95% CI: 78%–88%) in the case of prevalence higher than o75%.12 In our series, prevalence of malignancy was 89%. Therefore, the sensitivity obtained for bronchoscopy guided with fluoroscopy and EBUS, which was 76.6%, would be very close to the results reported in previously published studies.
Performance of fluoroscopy-guided bronchoscopy is known to depend on the size of the lesion.5–7 In this regard, Rivera and Metha13 reported a diagnosis yield of 63% for fluoroscopy-guided bronchoscopy in lesions with a diameter greater than 20mm, versus 34% in smaller lesions. Although lesion size is also a factor that determines performance in radial probe EBUS, a sensitivity of 71% (95% CI: 66%–75%) has been shown for lesions smaller than 25mm, and of 75% (95% CI: 70%–79%) for those with a mean size of at least 25mm.12 As for lesion location, most studies do not show significant differences.14,15 However, some authors indicate a worse yield when the lesions are located in the upper lobes, specifically in the apical and posterior segments of the left upper lobe, due to difficulties in inserting the ultrasound probe.11 Indeed, one limitation of radial probe EBUS lies in the difficulty in inserting the ultrasound ultra-miniprobe in the subsegmental bronchus dependent on the lesion. For this reason, access to the peripheral lesion was not possible in 8 patients in our series. We do not consider them false negatives, since the technique was not actually used. In this regard, access may be achievable with the use of a steerable guidewire (CC-6DR-1 Guiding Device; Olympus). Unfortunately, this device was not available in this center when the study was carried out.
No differences in the sensitivity of the method were demonstrated in the meta-analysis of Steinfort et al.12 when fluoroscopy was used in addition to radial probe EBUS: the rate for both groups was 73% (95% CI: 68%–78%). Although radial probe EBUS without fluoroscopy has proved useful for diagnosing smaller PPLs, its yield is somewhat less than that of radial probe EBUS with fluoroscopy.10,16 While both diagnostic techniques and localization cannot be performed simultaneously with ultrasound, fluoroscopy allows the bronchoscopist to ensure that samples are taken from the same area. Use of a guidewire, as reported in this paper, ensures that there is no displacement due to coughing or breathing movements by the patient. Moreover, the use of EBUS in our study did not increase exposure to fluoroscopy.
Our results are similar to those obtained in previous studies,12 although the differences did not reach statistical significance. This may be due to the sample size and the relatively good results obtained with the use of fluoroscopy-guided bronchoscopy (69.5%), thanks to the research team's long experience with this technique. The diagnostic yield of EBUS with fluoroscopy in lesions smaller than 30mm is really high, but we must remember that in our series there were only 10 patients in this subgroup. The bronchus sign is something to take into account whenever access by fiberoptic bronchoscopy is considered.17 Presence or absence of a bronchus sign was determined by the bronchoscopists themselves and not by expert radiologists. This explains why it could not be determined in all cases (in 47 of the 50 patients in the group undergoing fluoroscopy with radial probe EBUS, and in 77 of the 95 in the fluoroscopy group). In this series, no significant differences were found in terms of diagnostic yield when comparing the existence of bronchus sign. In any case, presence of bronchus sign does not always imply that the techniques are diagnostic, as there is the possibility that the lining may not be infiltrated. In this respect, carrying out a transbronchial needle aspiration may increase yield in these cases. Assessment by the radiologist or the use of more modern techniques, such as virtual bronchoscopy or other endobronchial navigation systems, might possibly provide results more aligned with the literature.18
Lesion identification by radial probe EBUS and the position of the ultrasound miniprobe in or adjacent to the lesion are some of the most important factors that determine its sensitivity.11,14,15,19 In this study, diagnostic yield reached 95% when this was achieved, compared to 25% in those patients in whom it was not possible.
In a recent study20 with a similar approach and a similar number of patients, limited by not being randomized, results comparable to those of this study were obtained. The overall diagnostic yield was 82.5% with fluoroscopy with radial probe EBUS, and 57.9% with fluoroscopy. In lesions smaller than 20mm, yields were 79.3% and 33.3%, respectively. As in the present study, etiology and lesion size did not seem to affect yield in the fluoroscopy with radial EBUS group, but did affect it in the fluoroscopy group.
Unlike fluoroscopy, which localizes the lesion only two-dimensionally, radial probe EBUS allows characterization and confirmation of lesion location from analysis of its echostructure, which is useful for differentiating between benignity and malignancy,21 and for deciding where the diagnostic techniques should be informed.
In conclusion, radial probe EBUS associated with fluoroscopy is a technique that is particularly useful for the diagnosis of smaller PPLs. It is safe for the patient, and it does not increase the total time of exposure to radiation inherent to fluoroscopy.
Conflicts of InterestThe authors declare no conflicts of interest.
The authors are grateful to Prof. Heinrich D. Becker, for all the knowledge transmitted and for his great humanity, and to the whole bronchoscopy team of Thoraxklinik in Heidelberg for their kind hospitality.
Please cite this article as: Sánchez-Font A, Giralt L, Vollmer I, Pijuan L, Gea J, Curull V. Utilidad de la ultrasonografía endobronquial radial en el diagnóstico de lesiones pulmonares periféricas. Estudio controlado con fluoroscopia. Arch Bronconeumol. 2014;50:166–171.