To investigate the etiology of pleural effusions (PE) in adults and the accuracy of pleural fluid (PF) cytology and cultures in malignant and infectious PE, respectively.
Patients and methodsRetrospective analysis of all consecutive patients with PE undergoing diagnostic thoracentesis during the last 19 years in a university hospital.
ResultsThe leading causes of PE among the 3077 patients were cancer (27%), heart failure (21%), pneumonia (19%), tuberculosis (9%), abdominal surgery (4%), pericardial diseases (4%) and cirrhosis (3%). Tuberculosis was the most common etiology in patients <34 years of age (52%), whereas heart failure predominated in octogenarians (45%). The most common primary tumors in malignant PE were lung (37%) and breast (16%) tumors. The overall accuracy of PF cytology was 59%, although it was significantly lower in mesotheliomas (27%) and squamous cell lung cancer (25%). In infectious PE, only 30% of cultures yielded positive results, a percentage which increased two-fold (66%) in purulent fluids (empyemas). Viridans streptococci were the most commonly isolated pathogens (25.5%). The sensitivity of solid media cultures of PF for Mycobacterium tuberculosis was low (18.5%).
ConclusionsThree quarters of patients with PE in whom a diagnostic thoracentesis was indicated had cancer, heart failure, pneumonia or tuberculosis. PF cytology and cultures give false negative results in a significant number of cases.
Conocer la etiología del derrame pleural (DP) en pacientes adultos y la rentabilidad de la citología y del cultivo de líquido pleural (LP) en DP malignos e infecciosos, respectivamente.
Pacientes y métodoEstudio retrospectivo de todos los pacientes consecutivos con DP sometidos a una toracocentesis diagnóstica durante los últimos 19 años en un hospital universitario.
ResultadosLas principales causas de DP en los 3.077 pacientes estudiados fueron: cáncer (27%), insuficiencia cardiaca (21%), neumonía (19%), tuberculosis (9%), cirugía abdominal (4%), enfermedades del pericardio (4%) y cirrosis (3%). La tuberculosis fue la etiología más común en pacientes<34 años (52%), mientras que la insuficiencia cardiaca lo fue en octogenarios (45%). Entre los DP malignos, los tumores primarios más comunes fueron el de pulmón (37%) y el de mama (16%). La citología del LP tuvo una rentabilidad global del 59%, pero fue significativamente inferior en mesoteliomas (27%) y carcinomas escamosos de pulmón (25%). En pacientes con DP infecciosos, solo el 30% de los cultivos del LP resultaron positivos, un porcentaje que se duplicó (66%) cuando el líquido era purulento (empiemas). Los estreptococos del grupo viridans representaron el 25,5% del total de aislamientos. El cultivo del LP en medio sólido para Mycobacterium tuberculosis tuvo escasa sensibilidad (18,5%).
ConclusionesLas 3 cuartas partes de los pacientes con un DP en los que se indica la realización de una toracocentesis diagnóstica tienen una neoplasia, insuficiencia cardiaca, neumonía o tuberculosis. La citología y los cultivos del LP son falsamente negativos en un porcentaje significativo de casos.
Pleural effusion (PE) is a common problem in patients seen in internal medicine and pneumology departments.1 Etiology can vary according to geographical area, healthcare setting, patient age or the time period studied, among other factors. Knowledge of the main etiologies of PE (pre-test probability) enables clinicians to correctly choose and interpret different diagnostic tests following a Bayesian clinical reasoning model.
Only 3 studies on the etiology of PE (with 484,2 6423 and 10004 patients) have been published in Spain in the past 25 years; the last of these was published more than a decade ago.4 The aim of this study was to describe the causes of PE and the accuracy of some basic tests such as pleural fluid (PF) cytology and culture in over 3000 consecutive patients diagnosed in a university hospital over the past 19 years.
Patients and MethodsOur hospital created a database in which the demographic and clinical characteristics of all consecutive adult patients undergoing diagnostic thoracentesis since 1994 have been entered prospectively, irrespective of the medical or surgical service from which they were referred. Most procedures were performed on hospitalized patients, and very few on outpatients. In this study, we analyze the etiologies of PE from January 1994 to June 2013. In total, 206 patients that had either no definite diagnosis or insufficient PF testing, imaging and clinical follow-up to establish a presumptive diagnosis or to consider the PE idiopathic was excluded. Each patient was included only once if 2 or more thoracenteses were performed for the same reason, i.e., to determine the etiology of PE. The data collection protocol was approved by the local ethics committee.
Diagnostic CriteriaHeart failure was diagnosed based on the patient's history, physical examination, chest X-ray, ECG, echocardiogram, if available, and response to diuretic therapy.
Patients were given a definitive diagnosis of malignant PE when PF cytology or pleural biopsy culture showed malignant cells. A diagnosis of probable malignancy was also accepted in patients with a known primary tumor, cytology-negative PE, and when other potential causes of PE had been ruled out.
A diagnosis of tuberculous PE was based on isolation of the tuberculosis bacillus in sputum samples, PF or pleural biopsy, or when the latter showed granuloma. Probable diagnosis was given in patients with lymphocytic exudate and pleural fluid adenosine deaminase of over >35U/L that was resolved with empirical antituberculosis therapy.
PE was considered parapneumonic when associated with pneumonia, bronchiectasis or pulmonary abscess. The effusion was classified as uncomplicated parapneumonic PE when it was resolved with antibiotic therapy alone. Complicated parapneumonic PE were those that could not be resolved without complete drainage of non-purulent PF, insertion of a pleural catheter or surgery. The term empyema means the presence of pus in the pleural space and is, by definition, classified as complicated PE even though it is not always associated with pneumonia.
A diagnosis of pulmonary embolism was dependent on a positive CT angiography or ventilation perfusion scan performed in a suitable clinical setting. Hepatic hydrothorax was considered in all cirrhosis patients who had no heart, infectious or malignant disease that would otherwise explain the PE. Idiopathic PE cases were those in which the etiology of the condition could not be determined despite thorough testing, and which resolved during follow-up. Widely accepted clinical criteria were applied to diagnose other diseases. PE were classified as exudative or transudative according to Light's criteria cases.5
Statistical AnalysisQuantitative variables are expressed as percentages, and qualitative variables as medians (25th and 75th percentiles) The chi-square and Kruskal–Wallis tests, with post hoc analysis of standard residuals, or the Mann–Whitney test was used as required to compare variables from the different PE etiology groups. P values of <.05 were considered statistically significant. The data were analyzed using SPSS version 18.0 (Chicago, IL, USA).
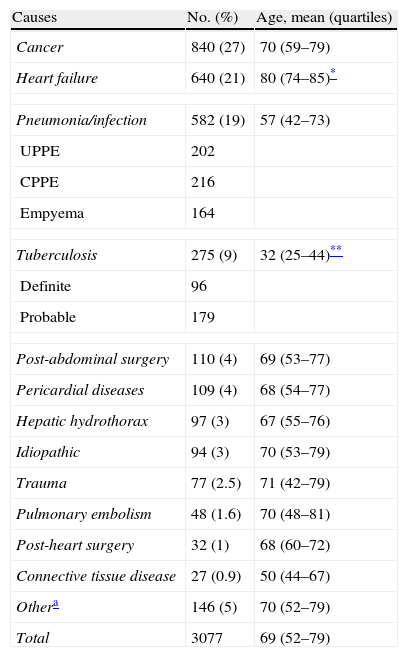
ResultsCauses of Pleural EffusionA total of 3077 patients with PE were included in the study. The mean age was 69 (52–79) years; 1826 (59%) were men and 1251 (41%) were women. In total, 75% of PE were due to one of the following 4 causes (Table 1): cancer (27%), heart failure (21%), pneumonia (19%) or tuberculosis (9%). These were followed, in terms of frequency, by post-abdominal surgery PE (4%), pericardial disease (4%) and hepatic hydrothorax (3%). Although gender did not significantly affect the etiology of PE, compared with other etiologies patients with tuberculous PE were significantly younger (median age 32 years), and those with heart failure were significantly older (80 years of age). As for older patients, the most common causes of PE in 760 octogenarians were heart failure (344; 45%), cancer (181; 24%) and pneumonia (86; 11%). At the other end of the scale, meanwhile, causes of PE in 293 subjects <34 years old were mainly tuberculosis (153; 52%) and pneumonia (75; 26%).
Etiology of Pleural Effusion.
| Causes | No. (%) | Age, mean (quartiles) |
| Cancer | 840 (27) | 70 (59–79) |
| Heart failure | 640 (21) | 80 (74–85)* |
| Pneumonia/infection | 582 (19) | 57 (42–73) |
| UPPE | 202 | |
| CPPE | 216 | |
| Empyema | 164 | |
| Tuberculosis | 275 (9) | 32 (25–44)** |
| Definite | 96 | |
| Probable | 179 | |
| Post-abdominal surgery | 110 (4) | 69 (53–77) |
| Pericardial diseases | 109 (4) | 68 (54–77) |
| Hepatic hydrothorax | 97 (3) | 67 (55–76) |
| Idiopathic | 94 (3) | 70 (53–79) |
| Trauma | 77 (2.5) | 71 (42–79) |
| Pulmonary embolism | 48 (1.6) | 70 (48–81) |
| Post-heart surgery | 32 (1) | 68 (60–72) |
| Connective tissue disease | 27 (0.9) | 50 (44–67) |
| Othera | 146 (5) | 70 (52–79) |
| Total | 3077 | 69 (52–79) |
CPPE: complicated parapneumonic pleural effusion; UPPE: uncomplicated parapneumonic pleural effusion.
Over the 19-year study period we observed a steady increase in heart failure (29% of PE from 2011 vs 15% prior to 2000) and a reduction in the number of cases of tuberculosis (5% of PE from 2011 vs 13% prior to 2000).
Causes of Exudative and Transudative EffusionsSufficient data were available to permit the application of Light's criteria in 2981 patients, of which 2316 (78%) had exudative and 665 (22%) transudative effusions. Of the former group, 797 (34.4%) had malignant PE, 542 (23.4%) parapneumonic PE, 269 (11.6%) tuberculous DP, and 154 (6.6%) heart failure. Transudate was mainly due to heart failure (485; 73%) and cirrhosis of the liver (87; 13%).
Light's criteria for exudative effusions were met by all (100%) tuberculous PE, post-traumatic effusion, pulmonary embolism and connective disease cases, 99% of pneumonia cases, 98% of malignant PE cases, 97% of post-heart surgery PE cases, 94% of PE secondary to pericardial disease and idiopathic PE, 24% of heart failure cases, and 9% of hepatic hydrothorax.
Radiological Aspects of Pleural EffusionOf the 2767 patients for whom posteroanterior chest X-ray data were available, 423 (15%) had massive PE occupying two-thirds or more of the hemithorax. Of these, most had malignant PE (244; 58%), pneumonia (72; 17%), tuberculosis (36; 8.5%) and hepatic hydrothorax (27; 6.4%). The percentage of massive PE due to these etiologies was 32%, 14%, 14%, and 34%, respectively.
Of a total of 2958 evaluable patients, chest X-rays showed bilateral PE in 667 (23%) cases. The most common causes were heart failure (357; 53.5%), cancer (121; 18%) and pericardial disease (47; 7%). Considering each etiology individually, 58% of heart disease, 47% of pericardial diseases and 41% of connective tissue diseases were associated with bilateral PE, while this characteristic was only detected in 15% of malignant and pulmonary embolism PE, in 8% of cirrhosis, in 6% of pneumonia, and in 4% of tuberculosis cases.
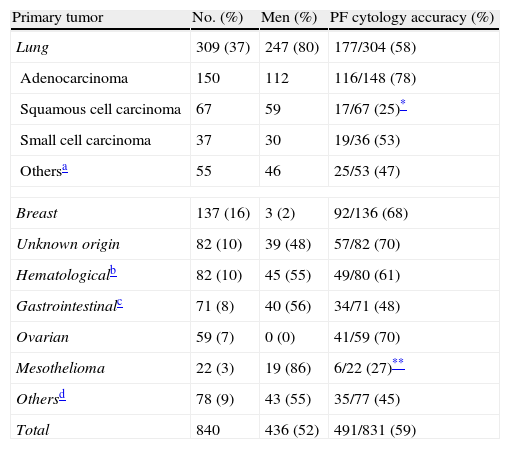
Malignant Pleural EffusionCytological or histological confirmation was obtained in 538 (64%) of 840 patients with malignant PE, while 302 (36%) of the remaining cases were considered probably malignant on the basis of the previously established criteria. All mesotheliomas were diagnosed using histological criteria. In approximately half of all malignant PE patients, the primary tumor was located in the lung (37%) or the breast (16%), as can be seen in Table 2. Prevalence of primary pleural tumors (mesotheliomas) was low in our region (3% of all malignant PE). Both lung tumors and mesotheliomas predominated in male subjects (80% and 86% of cases, respectively). At least half of the metastatic pleural tumors were adenocarcinomas.
Causes of Malignant Pleural Effusion and Accuracy of Pleural Fluid Cytology.
| Primary tumor | No. (%) | Men (%) | PF cytology accuracy (%) |
| Lung | 309 (37) | 247 (80) | 177/304 (58) |
| Adenocarcinoma | 150 | 112 | 116/148 (78) |
| Squamous cell carcinoma | 67 | 59 | 17/67 (25)* |
| Small cell carcinoma | 37 | 30 | 19/36 (53) |
| Othersa | 55 | 46 | 25/53 (47) |
| Breast | 137 (16) | 3 (2) | 92/136 (68) |
| Unknown origin | 82 (10) | 39 (48) | 57/82 (70) |
| Hematologicalb | 82 (10) | 45 (55) | 49/80 (61) |
| Gastrointestinalc | 71 (8) | 40 (56) | 34/71 (48) |
| Ovarian | 59 (7) | 0 (0) | 41/59 (70) |
| Mesothelioma | 22 (3) | 19 (86) | 6/22 (27)** |
| Othersd | 78 (9) | 43 (55) | 35/77 (45) |
| Total | 840 | 436 (52) | 491/831 (59) |
PF: pleural fluid.
Includes unknown (34), non-strain specific non-small cell (18), non-differentiated (2) and sarcoma (1).
Includes non-Hodgkin's lymphoma (61), leukemia (9), myelodysplastic syndromes (5), multiple myeloma (5) and Hodgkin's lymphoma (2).
Includes colon (25), stomach (22), pancreas (15), esophagus (5), bile duct (3) and gastrointestinal stromal tumor (1).
Overall, preliminary PF cytology was positive in 424 (51%) of 831 patients with malignant PE. A second test was performed on 214 patients with a preliminary cytologically negative effusion, and a positive result was obtained in 55 (26%) cases. Finally, in 52 patients with two negative cytologies, a third sample yielded positive results in 12 (23%) cases. On the whole, considering all the cytologies performed, the accuracy of this test in identifying malignancy was 59% (491/831). PF cytology was significantly less successful in identifying mesotheliomas (27%) and squamous cell lung cancer (25%).
Parapneumonic Pleural EffusionPF culture was performed on 562 parapneumonic and empyema PE cases, and was positive in 108 (66%) of 163 empyemas, 47 (23%) of 208 complicated parapneumonic PE, and 14 (7%) of 191 uncomplicated parapneumonic PE cases. In total, 18% of empyemas were not associated with pneumonia but with other processes such as esophageal perforation, post-surgical complications or spontaneous bacterial empyema in cirrhosis patients.
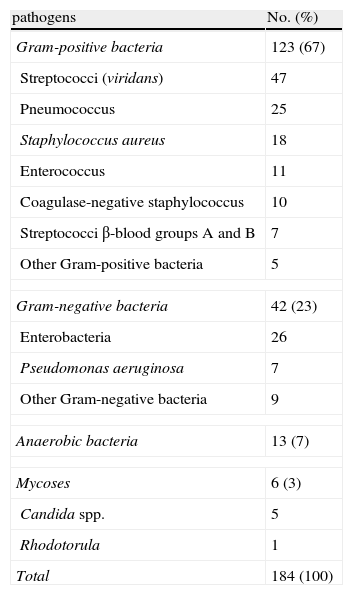
A total of 184 germs were isolated in 169 patients (Table 3); 156 cultures yielded a single pathogen, 11 had two pathogens and 2 had three pathogens. Viridans streptococci were the most commonly isolated pathogens both individually (39, 25%) and in polymicrobial cultures (9 of 13 cultures). In terms of purulent discharge, anaerobic bacteria (85%), enterococci (82%) and viridans streptococci (77%) were isolated in a significantly higher proportion than other bacteria (P=.03).
Microbial Isolates in Pleural Fluid.
| pathogens | No. (%) |
| Gram-positive bacteria | 123 (67) |
| Streptococci (viridans) | 47 |
| Pneumococcus | 25 |
| Staphylococcus aureus | 18 |
| Enterococcus | 11 |
| Coagulase-negative staphylococcus | 10 |
| Streptococci β-blood groups A and B | 7 |
| Other Gram-positive bacteria | 5 |
| Gram-negative bacteria | 42 (23) |
| Enterobacteria | 26 |
| Pseudomonas aeruginosa | 7 |
| Other Gram-negative bacteria | 9 |
| Anaerobic bacteria | 13 (7) |
| Mycoses | 6 (3) |
| Candida spp. | 5 |
| Rhodotorula | 1 |
| Total | 184 (100) |
Tuberculous pleural effusion was clinically diagnosed in 65% of cases. Mycobacterium tuberculosis was only found in 44 (18.5%) of 237 PF samples using Lowenstein–Jensen solid media culture. A total of 15 pleural biopsies were performed in patients with negative PF culture, and caseating granulomas were found in 13 of these cases. In patients with pleural tuberculosis, 78% (175/225) tested positive for tuberculosis, and 12.6% (17/135) were co-infected with HIV.
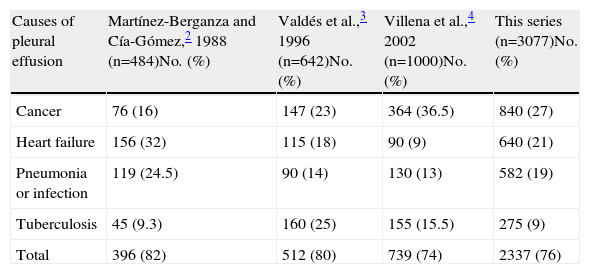
DiscussionThis study shows that in 75% patients undergoing diagnostic thoracentesis, the etiology of PE was, in order of frequency, cancer, heart failure, pneumonia and tuberculosis. These findings are consistent with previous Spanish series2–4 (Table 4), although the relative frequency of these 4 conditions varies. Specifically, a higher percentage of heart failure patients are found in a series taken from internal medicine departments,2 since this is one of the diagnosis-related groups most frequently seen by these specialists. Pleural tuberculosis etiology predominates in Spanish regions with a greater prevalence of this disease, such as Galicia,3 although the number of cases has steadily diminished over the past 10 years,6 a trend that was also reflected in our series.
Spanish pleural Effusion Series Over the Past 25 Years.
| Causes of pleural effusion | Martínez-Berganza and Cía-Gómez,2 1988 (n=484)No. (%) | Valdés et al.,3 1996 (n=642)No. (%) | Villena et al.,4 2002 (n=1000)No. (%) | This series (n=3077)No. (%) |
| Cancer | 76 (16) | 147 (23) | 364 (36.5) | 840 (27) |
| Heart failure | 156 (32) | 115 (18) | 90 (9) | 640 (21) |
| Pneumonia or infection | 119 (24.5) | 90 (14) | 130 (13) | 582 (19) |
| Tuberculosis | 45 (9.3) | 160 (25) | 155 (15.5) | 275 (9) |
| Total | 396 (82) | 512 (80) | 739 (74) | 2337 (76) |
The causes of PE in patients at either end of the age scale differ significantly. While the cause of approximately half of all PE cases among young patients (<34) is tuberculosis, among octogenarians PE is secondary to heart failure, which is consistent with the findings of Villena et al.4 It is important to note that the higher prevalence of pericardial (4%) and hepatic hydrothorax (3%) etiologies in our study compared with previous series3,4 is probably due to the fact that these studies recruited, either solely or chiefly, pulmonology patients.
Distinguishing between transudative and exudative effusion is traditionally considered a fundamental first step in the diagnosis of PE. In clinical practice, these are distinguished using Light's criteria.5 Most transudates are associated with heart failure (75%–80%),2–4 although pericardial effusion meets Light's criteria for exudate, albeit narrowly, in around 25% of cases.7 Contrary to the belief that pulmonary embolism can produce transudative PE, we agree with other authors8 who claim that PE due to pulmonary embolism always meets Light's criteria for exudative effusion.
As far as radiological evidence is concerned, differential diagnosis is further refined by the presence of bilateral PE or effusion clouding almost the entire hemithorax. Cancers cause over half of massive PE,9 and heart failure causes over half of bilateral PE. Occasionally, bilateral PE can have a different causative factor for each side, a condition known as Contarini's syndrome.10
In both this and other series published,2–4,11 lung cancer is responsible for a third or more of malignant PE. Experts, with a few exceptions,4 also agree that around 10% of malignant PE are associated with an unknown primary tumor.2,3,11,12 However, the relative frequency of mesotheliomas varies according to geographical region, in relation to the risk of asbestos exposure. Therefore, while mesotheliomas in ours and other series2,3 account for only 3% of all malignant PE, this increases by four- or five-fold (13%–14.5%) in other Spanish studies.4,12 In line with the literature,12,13 overall accuracy of PF cytology in diagnosing malignancy was found to be approximately 60%. Nevertheless, it is important to note that accuracy was considerably lower in squamous cell lung cancer and mesotheliomas (25% and 75%, respectively). Mesothelioma is a prime example of how the combination of an experienced cytologist (in parallel to the prevalence of the disease) and the use of immunocytochemistry can significantly improve the accuracy of PF cytology. These factors were combined in the study conducted by the Australian group Segal et al. who recently reported a PF cytology sensitivity of 73% in 517 cases of mesothelioma.14 Finally, the diagnostic yield of PF cytology for hematological malignancies (61%) reported in our study contrasts with the findings of another Spanish group (18%).12 However, this could be due to the fact that in our hospital it is common practice to request a flow cytometric analysis of PF whenever a lymphoma is suspected.
With regard to PF microbiology, in our study only 30% of patients with infectious PE tested positive using standard PF culture, a percentage that increases to 60% in the case of purulent fluid (empyema). Two large studies have also reported positive results in 56.4% and 58% of 40615 and 43416 empyemas, respectively. In our series, streptococci accounted for 43% of all isolates, the most common being viridans (formerly milleri), which accounted for 25.5% of all positive cultures, a finding that is consistent with the results of Maskell et al. (24% of 336 isolations in community-acquired empyemas).16 Anaerobic bacteria isolation is dependent not only on correct collection, transport and processing procedures, but also on the culture medium used. These bacteria made up 7% of the isolates in our series, compared with 5.7%,15 8.5%,17 and 20%16 reported in three other large studies. Of course, inoculating PF into blood culture bottles18 or the use of nucleic acid amplification techniques19 can significantly increase the number of bacteria isolated. With regard to pleural tuberculosis, we were only able to isolate Mycobacterium tuberculosis from PF using the Lowenstein–Jensen method in 18.5% of cases, a percentage well within the 7%20 to 36%21 range described in the literature. The low yield in this case is due to the fact that tuberculous pleuritis is a paucibacillary disease. However, yield can be greatly increased by using liquid culture media (63% sensitivity in a series of 382 pleural tuberculosis cases).22
This study has certain limitations. Only patients undergoing diagnostic thoracentesis were included. This could mean that some PE etiologies are poorly represented, such as heart failure (many patients presenting with characteristic symptoms are diagnosed with pericardial effusion and do not undergo thoracentesis) or pulmonary embolism (effusion is generally insignificant and the patient is usually given anticoagulants while awaiting confirmatory lab results). In addition, the lack of cardiothoracic surgery services in our hospital could explain the paucity of PE cases associated with these procedures.
In conclusion, cancer, pneumonia and tuberculosis are the causes of most pleural exudates, while heart failure is the main cause of transudates. Pericardial and hepatic hydrothorax conditions are also relatively frequent causes of PE. Pleural tuberculosis predominates in young adults, while in octogenarians the main etiology is heart failure. The bacterial yield from infectious pleural effusion culture is low, except in the case of purulent PF. Likewise, solid medium culture identifies very few cases of tuberculous PE. PF cytology is a simple method for diagnosing malignancies, although it can yield false negatives in at least 40% of cases.
FundingNo funding was received for this study.
Conflicts of InterestThe authors have no conflicts of interest.
We thank the biobank of the Institut de Recerca Biomèdica in Lleida, part of the Xarxa Catalana de Bancs de Tumors and RETIC Biobancos RD09/0076/00059, for processing and storing pleural fluid samples.
Please cite this article as: Porcel JM, Esquerda A, Vives M, Bielsa S. Etiología del derrame pleural: análisis de más de 3.000 toracocentesis consecutivas. Arch Bronconeumol. 2014;50:161–165.