Massive hemoptysis is a medical emergency that involves multiple diagnostic and therapeutic challenges. Unlike bleeding from other sources, a small amount of blood can quickly occlude the airway, causing severe hypoxemia. Initial management should aim to stabilize the patient, secure the airway, and isolate the focus of the bleed. The optimal etiological treatment will then be determined (various bronchoscopic techniques, embolization, etc.).1 We report an uncommon and extreme case of a patient with advanced heart disease and pulmonary hypertension in whom hypoxemia led to rapid circulatory and respiratory collapse, advising against the implementation of commonly used protocols and procedures, given the imminent risk of cardiac arrest.
Our patient was a 62-year-old man with hypertrophic cardiomyopathy who had undergone myectomy, mitral plasty, and mechanical aortic prosthesis 5 years previously. He was admitted to our hospital for acute pulmonary edema, with normally functioning prostheses, LVEF 52%, pulmonary hypertension, and restrictive filling. He developed cardiogenic shock requiring orotracheal intubation, intra-aortic balloon counterpulsation (IABC), norepinephrine 0.5 µg/kg/min, and dobutamine 8 µg/kg/min. In the following hours, the patient’s progress was favorable, and IABC and vasoactive drugs could be withdrawn.
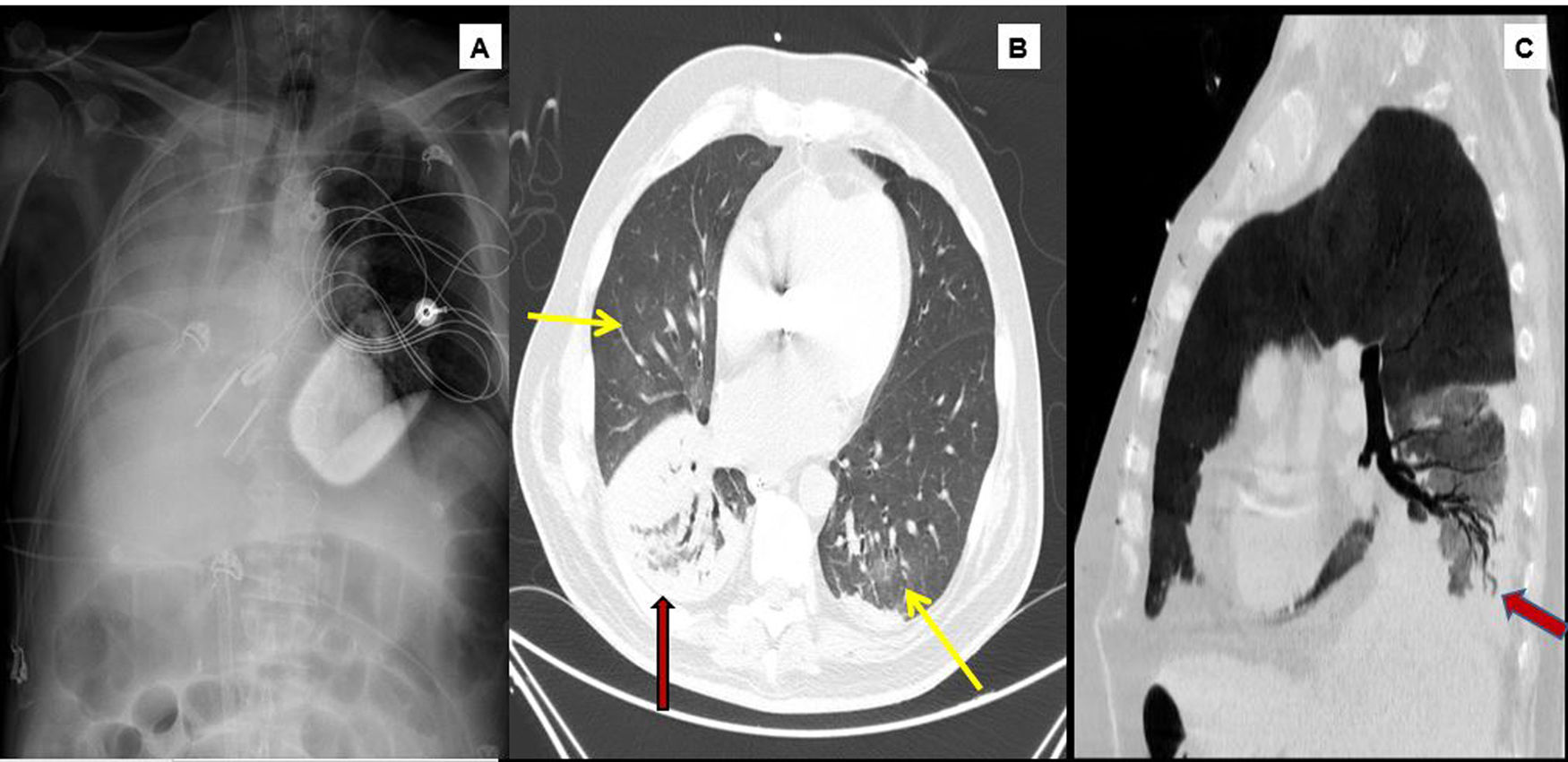
However, his subsequent course was marked by respiratory worsening with alveolar opacities on the right lung base, fever, and raised acute phase reactants despite favorable hemodynamic, echocardiographic and NTproBNP values. Although microbiological results were not yet available, these data, together with a favorable response to wide spectrum empirical antibiotics, supported the suspicion of right lower lobe pneumonia associated with mechanical ventilation. The infectious disease was beginning to resolve, but then, on day 7 of admission, the patient developed a sudden desaturation of up to 85% despite increasing FiO2 to 100%. Auscultation revealed disseminated rhonchi with marked hypoventilation of the right hemithorax, and blood clots were aspirated through the orotracheal tube. An urgent bedside chest X-ray showed atelectasis of the right lower lobe with bilateral alveolar opacities in the rest of the parenchyma. The patient presented rapidly progressing hypoxemia within the next few minutes (minimum PaO2 32 mmHg) that did not improve with right lateral decubitus Ambu ventilation, along with hypotension and hyperlactacidemia (lactate 5.1 mmol/L) refractory to the administration of increasing doses of volume expanders and vasopressors. Given the immediate risk to life, we decided not to perform fiberoptic bronchoscopy and ruled out the option of selective orotracheal intubation. We therefore prioritized respiratory and circulatory stabilization, using ultrasound guided cannulation to establish venovenous ECMO at the bedside within a few minutes, without sodium heparin, by placing a 23 F afferent cannula via the right femoral vein and a 17 F efferent cannula via the right jugular artery. This procedure left us with the option of subsequently adding an arterial cannula for hemodynamic support in the form of venoarterial ECMO if necessary. Oxygenation was immediately normalized after implantation, and hemodynamics improved gradually. We could then proceed with diagnostic fiberoptic bronchoscopy that showed the presence of fresh blood in the left bronchial tree and hyperemic mucosa with no underlying injury after aspiration, and a cast of thrombotic material in the right pulmonary tree that was impossible to extract completely. Fig. 1 shows the chest X-ray after ECMO cannulation confirming an sufficient distance between the cannulas to prevent recirculation phenomena, the disappearance of left hemitorax condensations, and persistent right pulmonary atelectasis where the presence of a clot in the bronchial lumen within a few centimeters of the carina is observed.
1A) Chest X-ray. Subtotal atelectasis of the right lung, ECMO ejection cannula in the right jugular and suction cannula in the inferior vena cava. Contrast computed tomography (arterial phase, lung window). 1B) Predominantly central ground glass infiltrates (yellow arrows) observed in both lung fields. Consolidation in right lower lobe (red arrow). 1C) Permeability of the bronchial tree observed in the center of the consolidation focus (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Support was maintained with ECMO at a flow of 4 L/min and FiO2 for PaO2 > 60 mmHg, under protective ventilation, without anticoagulation. Daily bronchoscopies were performed until the thrombotic material was extracted in its entirety. After 4 days on ECMO, reexpansion of the right lung was achieved with no new bronchial bleeds. Tests to disconnect venovenous ECMO were then undertaken and the patient was subsequently decannulated. This was followed by a computed tomography scan with contrast in the arterial and venous phases that detected an image of right basal condensation with no evidence of bleed foci in the bronchial circulation, fistulas, cavitations or other lesions (fig. 2). Hemoptysis was eventually attributed to bleeding associated with pneumonia in a patient with pulmonary hypertension receiving anticoagulation. Progress in the following weeks was gradual, and the patient was discharged 69 days after admission.
The number of indications for ECMO are increasing steadily. This treatment can be coupled with cardiorespiratory support in severe situations, permitting subsequent investigation and treatment of the cause. The main indication for venovenous ECMO is respiratory distress; however, it can also be beneficial in other settings, such as status asthmaticus, airway obstruction, or massive pulmonary hemorrhage.2,3 Rescue with venovenous ECMO has only been mentioned briefly in recent reviews of massive hemoptysis,1 yet ECMO is a useful tool for stabilizing the situation prior to the usual sequence of tests and therapies. Other hospitals have published successful results in similar contexts.4–6 The largest series is that of Kim et al.,7 who report 15 ECMO implants for airway obstruction, 5 of which were due to massive hemoptysis from different origins.
This case also illustrates the balance between hemorrhagic and thrombotic risk, since a patient with life-threatening hemoptysis could be managed for 4 days without anticoagulation using venovenous ECMO support at flows greater than 4 l/min, with close monitoring of prosthesis function. Venovenous ECMO support without anticoagulation may increase the incidence of thrombotic phenomena, mainly deep vein thrombosis, pulmonary thromboembolism and oxygenation membrane thrombosis, although this appears to be compensated by the use of high flows, which help reduce blood stasis.8 Evidence supporting the safety of venovenous ECMO without anticoagulation in selected patients appears to be growing.9 This strategy has already been described in patients with trauma or active bleeding, and both venovenous ECMO9 and venoarterial ECMO10 have even been used in patients with no particular hemorrhagic risk.
FundingNo funding has been received for this manuscript.
Please cite this article as: Martínez-Solano J, Sousa-Casasnovas I, Fernández MJ, Devesa-Cordero C, Fernández-Avilés F, Martínez-Sellés M. Canulación urgente a pie de cama y sin anticoagulación de membrana de oxigenación extracorpórea venovenosa en un paciente con hemoptisis masiva y shock refractario. Arch Bronconeumol. 2020;57:73–74.