Assessment of patients with suspected interstitial lung disease (ILD) includes surgical lung biopsy (SLB) when clinical and radiological data are inconclusive. However, cryobiopsy is acquiring an important role in the ILD diagnostic process. The objective of this study was to evaluate the diagnostic yield, safety, and economic costs of the systematic use of cryobiopsy in the assessment of patients with suspected ILD.
MethodsThis was a retrospective observational study of patients who had undergone transbronchial cryobiopsy for the evaluation of ILD from January 2011 to January 2014. The procedures were performed with a video bronchoscope using a cryoprobe for the collection of lung parenchyma specimens, which were analyzed by pathologists. Diagnostic yield, complications, and economic costs of this technique were analyzed.
ResultsCriobiopsy specimens from a total of 33 patients were included. A specific diagnosis was obtained in 26, producing a diagnostic yield of 79%. In 5 patients, SLB was required for a histopathological confirmation of disease, but the procedure could not be performed in 4, due to severe comorbidities. The most frequent complications were pneumothorax (12%) and grade I (9%) or grade II (21%) bleeding. There were no life-threatening complications. The systematic use of cryobiopsy saved up to €59.846.
ConclusionCryobiopsy is a safe and potentially useful technique in the diagnostic assessment of patients with ILD. Furthermore, the systematic use of cryobiopsy has an important economic impact.
En los pacientes con enfermedad pulmonar intersticial difusa (EPID) que presentan datos clínicos y radiológicos inconsistentes es recomendable la realización de una biopsia pulmonar quirúrgica (BPQ). La criobiopsia es una técnica endoscópica reciente menos invasiva que la BPQ que podría jugar un papel relevante en el diagnóstico de las EPID. El objetivo del presente estudio es analizar la rentabilidad diagnóstica, las complicaciones y los costes económicos derivados del uso de la criobiopsia en el diagnóstico de las EPID.
MétodosEstudio observacional retrospectivo en el que se incluyeron pacientes afectados de EPID tributarios de biopsia pulmonar, a los que se les practicaron criobiopsias desde enero de 2011 a enero de 2014. El procedimiento se realizó mediante videobroncoscopio, bajo anestesia general y ventilación mecánica. Se analizó la rentabilidad diagnóstica, las complicaciones producidas y los costes económicos derivados de esta técnica.
ResultadosSe analizaron las muestras de criobiopsia de un total de 33 pacientes. Se obtuvo un diagnóstico específico en 26, lo que representa una rentabilidad diagnóstica del 79%. Cinco pacientes hubieran requerido BPQ para confirmación histológica, aunque en 4 de ellos no pudo realizarse por presentar comorbilidades graves. Las complicaciones más frecuentes fueron el neumotórax (12%) y el sangrado moderado (21%). No hubo complicaciones graves. Considerando que a los pacientes con diagnóstico específico se les evitó una BPQ, la criobiopsia representó un ahorro económico estimado de hasta 59.846€.
ConclusionesLa criobiopsia es una técnica segura y potencialmente útil en el diagnóstico de las EPID que permite, además, un ahorro económico considerable.
The differential diagnosis of diffuse interstitial lung diseases (ILD) is a complex process, often requiring a multidisciplinary team and lung parenchyma sample collection using various diagnostic techniques. Idiopathic pulmonary fibrosis (IPF) is the most common disease within this group, and has the poorest prognosis1–3; whereas differential diagnosis with other ILDs presents a major challenge.
International consensus guidelines for the diagnosis and treatment of ILD1,2 indicate that in approximately 50% of cases, chest high-resolution computed tomography (HRCT) is sufficient to diagnose IPF in the correct clinical context.4,5 In the remaining patients, radiological criteria are inconclusive, and surgical lung biopsy (SLB) is recommended in order to distinguish usual interstitial pneumonia (UIP) from other ILDs.6,7 Transbronchial biopsy (TBB) via fiberoptic bronchoscopy (FBS) can provide diagnosis in some cases of ILD.8–11 This technique, however, has been dismissed in clinical guidelines1–3 because the frequently patchy nature of ILDs, small size of specimens obtained and poor preservation means that the diagnostic yield of TBB is at times less than 30%.12,13
Recent studies have shown that, unlike conventional TBB, TBB using a cryoprobe (also known as cryobiopsy) is a viable technique for obtaining lung parenchyma samples with a higher diagnostic yield and relatively few complications.14,15 It differs from conventional TBB in that samples for histological analysis are larger and better preserved.16–19 Furthermore, unlike SLB, it does not require an operating theater and can be performed on an outpatient basis. The role of cryobiopsy in the diagnostic algorithm of IPF and differential diagnosis with other ILDs is unclear, and its potential benefits have not been specifically determined in a cost–benefit analysis.
The main aim of this study was to analyze the diagnostic yield of cryobiopsy in the differential diagnosis of ILDs. As a secondary objective, we analyzed the complications and economic costs derived from the systematic application of cryobiopsy in ILDs.
MethodsPatientsThis was a retrospective observational study that analyzed the results of lung cryobiopsies performed in the Respiratory Endoscopy Unit of a tertiary hospital between January 2011 and January 2014. Patients had been previously assessed by an ILD multidisciplinary committee, where all cases referred from the different hospital departments, outpatients, and district primary care clinics were evaluated. Clinical decisions were taken by consensus among the members of the multidisciplinary team, which was composed of pulmonologists, thoracic surgeons, radiologists, and specialists in systemic and pathological autoimmune diseases.
Patients with an ILD pattern on HRCT defined as “possible UIP” or “inconsistent with UIP”, in whom histological confirmation would be required (in accordance with ATS/ERS guidelines1) were included. The specific lobe where the cryobiopsy was performed was determined following the radiologists’ recommendations, based on ATS/ERS1 criteria for surgical lung biopsy. In summary, lung biopsy should cover a wide spectrum of lung disease, including honeycomb foci, as this finding is a criterion for UIP. However, if there are signs of very severe fibrosis with gross honeycombing, the biopsy should be taken from less affected honeycomb areas, as non-specific changes are usually found in the most affected areas. If the lung does not show any signs of fibrosis or honeycombing, the sample should be taken from radiologically abnormal areas. Following this protocol, biopsies were taken in all cases from different sub-segments of the target lobe and, when possible, from various lobes. All patients had a complete blood count with coagulation study, echocardiography and lung function study that included forced spirometry, determination of lung volumes and carbon monoxide diffusing capacity. SLB was proposed in patients in whom the cryobiopsies were not diagnostic. All patients signed a specific informed consent for cryobiopsies.
ProcedureThe cryobiopsies were performed in an examination room equipped with hemodynamic monitoring equipment, a ventilator and mechanical radioscopy arm. The procedure was carried out under general anesthetic with propofol (3–6mg/kg/h) and remifentanil (0.05–0.1mg/kg/h) infusion. Patients were intubated with a flexible, cuffed tube (7.5mm Bronchoflex, Rüsch, Teleflex Medical, Durham, NC, US). This tube has a side channel that allows the introduction of a Fogarty™ occlusion balloon catheter (4F/5F/6F; Edwards Lifesciences Corporation™, Irvine, CA, US), which was placed at the entry of the lobe to be biopsied and inflated immediately after performing the cryobiopsy to stem any possible bleeding from the target airway. An EB-1970K video bronchoscope (outer diameter: 6.2mm, diameter of working channel: 2.8mm; Pentax Medical, US) was used. After examining the bronchial tree, bronchoalveolar lavage was carried out in the area selected by HRCT imaging in patients in whom it had not previously been performed. TBB was then performed on the selected segments using a 1.9mm cryoprobe (Erbokryo CA, Erbe, Germany) under fluoroscopic guidance. The cryoprobe was introduced through the working channel of the bronchoscope and, once the correct position had been confirmed by fluoroscopy, the cryoprobe pedal was activated for 3–4s. The probe was then withdrawn, together with the bronchoscope, with the specimen adhered to the tip of the cryoprobe. At least 3 samples were taken, although the total number of biopsies depended on their size, the patient's tolerance and possible complications. After the procedure had been completed and the patient ex-tubated, a post-procedure chest X-ray was performed. Any complications observed were recorded, particularly bleeding and pneumothorax. Pulmonary bleeding was classified according to its severity as: grade I: mild bleeding which does not require endoscopic intervention; grade II: moderate bleeding which stops within 3min after endoscopic intervention (bronchial occlusion and/or instillation of cold serum), and grade III: severe bleeding that cannot be controlled endoscopically, causing hemodynamic or respiratory instability, and making it necessary to halt the procedure.20
Histological EvaluationThe specimens were fixed in 4% formaldehyde and sent to the histopathology laboratory, where they were measured before being embedded in paraffin. Each sample was embedded in a separate block and stained with hematoxylin–eosin and Masson's trichrome stain. The specimens were evaluated by a pathologist from the multidisciplinary team with experience in respiratory disease pathology. The sample was not considered diagnostic when the findings did not allow a specific diagnosis to be made. Samples with insufficient alveoli for analysis were considered invalid.
Evaluation in the Multidisciplinary CommitteeThe clinical, radiological, and histopathological parameters were reviewed by specialists who regularly participated in our hospital's ILD multidisciplinary committee. A sample was considered to be diagnostic when the histopathological findings enabled the specific diagnosis of the particular disease. Once the histopathological report had been obtained, the members of the multidisciplinary committee established the definitive diagnosis of the patient's interstitial disease based on the report and the patient's clinical and radiological characteristics.
Economic AnalysisFor the economic analysis, the cost of the different diagnostic processes was calculated in euros. We used data based on the Catalonian Government taxes and public prices Act approved by Legislative Decree 33/2008 of 25 June and Act 26/2010 of 3 August, and data reported by other authors in the same setting.21,22 The final cost per process was calculated by totaling the cost of the different procedures required to reach the definitive diagnosis.
Statistical AnalysisThe results of the quantitative variables were expressed as mean±standard deviation. Percentages and absolute frequencies were used for the qualitative variables. Statistical data analysis was performed using the SPSS statistics package version 17.0 for Windows® (SPSS, Chicago, IL, US).
ResultsDiagnostic Yield and Safety of the ProcedureDuring the study period, 33 patients underwent lung cryobiopsy for confirmation of ILD. Their general characteristics are summarized in Table 1. The location of the biopsies, number of samples obtained, and complications observed are summarized in Table 2. The bronchoalveolar lavages were performed in the middle lobe or lingula to avoid technical difficulties in performing the cryobiopsy. More than one lung lobe was biopsied in 2 patients. In the remaining 31 patients, the biopsies were carried out in various segments of the lower lobes. A mean of 2.7 biopsies were obtained per patient, with a mean size of 0.4cm (SD±0.17). Biopsy specimens considered to be diagnostic were obtained in 26 patients (79%) (Fig. 1); the samples were considered non-diagnostic in 5 patients (15%) and invalid in only 2 patients (6%). There were no significant differences in the size of the diagnostic and non-diagnostic specimens (0.5±0.19cm and 0.34±0.15cm, respectively). In general, no artifacts due to compression of the alveoli were found in the samples analyzed. Fig. 2 summarizes the diagnostic orientation based on the HRCT images. The definitive diagnoses were obtained from the histopathology of the cryobiopsy samples and the opinion of the multidisciplinary team. Of the 5 patients with non-diagnostic specimens, a diagnostic SLB (peribronchiolar metaplasia) was performed in only 1 case (3%). Of the remaining 4 patients, 2 cases were diagnosed with idiopathic NSIP, mainly based on radiological criteria. Given that these patients had severe comorbidities that contraindicated the intervention, SLB was dismissed by the multidisciplinary team. The other 2 cases had radiological images, symptoms, and histopathological findings suggestive of ILD, but a more accurate diagnosis could not be reached, so they were diagnosed with ILD of unknown origin. Of the 2 patients with invalid samples, one had radiological images suggestive of organized pneumonia. Since SLB was also contraindicated due to severe comorbidities, the patient was treated with oral corticosteroids, with a good clinical and radiological response. In the other case, the radiological image and symptoms suggestive of ILD were mainly taken into account for the final diagnosis, suggesting ILD of unknown origin. In 1 patient, despite images characteristic of UIP on the HRCT, a cryobiopsy was performed due to clinical suspicion of hypersensitivity pneumonitis. Cryobiopsies were performed in patients with ILD associated with a systemic autoimmune disease (SAD) due to the lack of criteria for a firm diagnosis of SAD. These patients had lung disease at the outset and later presented extra-pulmonary symptoms, thus fulfilling sufficient criteria for a diagnosis of systemic sclerosis. As regards the safety of the technique, 4 cases of pneumothorax (12%) were identified; 3 resolved spontaneously while 1 required a chest drain for 2 days, after which the patient was discharged. In total, 9% of patients had grade I bleeding, and 21% had grade II bleeding. No cases of severe bleeding requiring suspension of the procedure were detected, and no additional medical or surgical measures were needed to control bleeding. Although 2 patients had mild pulmonary hypertension as comorbidity, neither of them had complications.
Characteristics of Patients Undergoing Lung Cryobiopsy.
| Characteristics | No. (%) or mean (range) |
|---|---|
| Age, years | 64 (30–79) |
| Sex | |
| Male | 11 (33) |
| Female | 22 (68) |
| Smoking habits | |
| Never | 16 (49) |
| Former smoker | 13 (39) |
| Active smoker | 4 (12) |
| BMI | 29 (22–37) |
| LFT pre-intervention (% reference) | |
| FVC | 69 (43–103) |
| FEV1 | 73 (49–97) |
| FEV1/FVC | 79 (66–92) |
| TLC | 73 (49–102) |
| DLCO | 50 (23–82) |
| DLCO/AV | 72 (41–94) |
| Home oxygen therapy | 1 (3) |
| Pre-intervention PH | 2 (6) |
DLCO, carbon monoxide diffusing capacity; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; PH, pulmonary hypertension; BMI, body mass index; LFT, lung function tests; TLC, total lung capacity; AV, alveolar volume.
Characteristics of the Procedure.
| Characteristics | No. (%) |
|---|---|
| Location of the biopsy | |
| RLL | 24 (73) |
| LLL | 7 (21) |
| LLL and RLL | 1 (3) |
| LLL and lingula | 1 (3) |
| Number of samples (median, range) | 2.7 (0–5) |
| Complications | |
| Mild bleeding | 3 (9) |
| Moderate bleeding | 7 (21) |
| Severe bleeding | 0 (0) |
| Pneumothorax | 4 (12)a |
RLL, right lower lobe; LLL, left lower lobe.
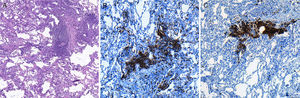
Optical microscopy image of a lung biopsy histological section, showing: (A) good conservation of the alveolar structures of the specimen, as well as the presence of predominantly lymphocytic inflammatory infiltrate located in the interstitium (hematoxylin-eosin, 40×). (B) The immunohistochemical study shows the presence of T lymphocytes (CD3 labeling; 100×) and (C) B lymphocytes (CD20 labeling, 100×). The patient was diagnosed with lymphocytic interstitial pneumonia, with no evidence of lymphoma.
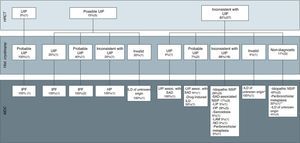
Final diagnoses made by the multidisciplinary committee based on the radiological images, classified according to ATS/ERS guidelines for idiopathic pulmonary fibrosis into usual interstitial pneumonia (UIP), possible UIP and inconsistent with UIP, and histopathological results of the cryobiopsy specimens.
Hist, histopathology; MCD, multidisciplinary committee diagnosis; SAD, systemic autoimmune disease; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; LAM, lymphangioleiomyomatosis; HP, hypersensitivity pneumonitis; LIP, lymphocytic interstitial pneumonia; NSIP, nonspecific interstitial pneumonia; UIP, usual interstitial pneumonia; OP, organized pneumonia; HRCT, high-resolution computed tomography. *Based on radiological images, the suspected diagnosis was OP. **The final diagnosis of peribronchiolar metaplasia was made by SLB.
Following the method used by Leiro Fernandez et al.22 and establishing the cost of FBS at €250.45, cryoprobe at €54 per case, and histopathology analysis at €253.69, the total cost of the cryobiopsies performed was [(€250.45+€54+€253.69)×33]=€18,426.54, giving a total cost of €304.69 per patient. In 26 of the 33 patients evaluated, the cryobiopsy provided the definitive diagnosis of the disease and SLB was not needed. The costs of this surgical technique in both the hospital and outpatient setting are summarized in Table 3. In the case of the 3 patients who presented pneumothorax with spontaneous resolution, the added cost was €1458.30 (€486.10 per patient). In the case of the patient who required a drainage tube to resolve the pneumothorax, the added cost was €2229.98. Therefore, the systematic use of lung cryobiopsy in the assessment of patients with suspected ILD reduced costs by €41,506.74−(€10,054.77+€3688.28)=€27,763.69 in the case of SLB without admission; €57,548.04−(€10,054.77+€3688.28)=€43,804.99 in the case of 24-h hospital stay, and €73,589.34−(€10,054.77+€3688.28)=€59,846.29 if hospital stay was extended to 48h.
DiscussionThis study shows that specimens obtained with cryobiopsy are of sufficient quality for specific diagnosis in most cases of suspected ILD when histological findings are combined with clinical, radiological, and laboratory data. Furthermore, this technique was shown to be safe and much less costly than SLB, and could be an alternative to consider in the diagnostic management of these patients.
It has been estimated that approximately one third of patients with ILD will require lung biopsy, although this will be performed in only 7.5%–12%,3,4,15 highlighting the reticence of some patients and physicians to perform this procedure. Moreover, although suitable samples for histopathological study can be obtained with SLB,23,24 it is not without significant drawbacks, such as the need for an operating theater and hospitalization in most cases, limited availability, and moderate morbidity and mortality.25–29 The recent introduction of the flexible cryoprobe, which thanks to the Joule–Thomson effect generates very low temperatures at its distal tip (around −85°C), enables larger, better preserved TBB samples to be obtained than those taken with forceps.14,30–33 In 2009, Babiak et al.16 published the first report on the potential usefulness of cryobiopsy in diffuse lung diseases. Although this study included a very heterogeneous group of patients, and the primary objective was not to determine diagnostic yield, the information provided by the cryobiopsy contributed substantially to the definitive diagnosis in a significant number of cases.
Casoni et al.19 evaluated 69 patients with undefined diffuse interstitial pneumonia, and obtained a specific diagnosis in 76% of cases. Very recently, Pajares et al.14 in a randomized study of 77 patients also showed that cryobiopsy obtained specific diagnoses in 74% of patients with interstitial disease, a significantly higher yield than the 34% obtained using traditional TBB. In our study, diagnostic yield was 79%, very similar to that of Casoni et al. and Pajares et al., confirming the usefulness of cryobiopsy in this patient group. Interestingly, the diameter of the probe used in our study (1.9mm) was smaller than that reported by Casoni et al. and Pajares et al. (2.4mm). Although there is experimental evidence that the size of the specimen depends, among other variables, on the diameter of the probe, the fact remains that the number of specific diagnoses does not appear to differ greatly according to the size of probe.30 Furthermore, the number of complications in our series was acceptable, the most notable being pneumothorax in 12% of cases, with moderate bleeding in 20%, and a 48-h chest drain in only 1 patient. The hemostatic effect of freezing the cryoprobe probably contributed to the low incidence of bleeding, which in no case required specific intervention or interruption of the procedure.
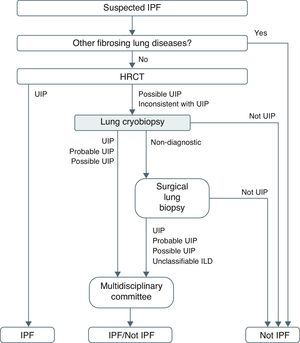
Although there are no studies comparing the yield of cryobiopsy and SLB, the former certainly appears to have advantages that make it worth considering in ILD diagnostic protocols (Fig. 3). This study also highlights an additional advantage of cryobiopsies over SLB: cost. Systematic cryobiopsies could save between €31,451.97 (€953.09/patient), in the case of outpatient SLB, and €59,846.29 (€1925.29/patient) in the case of SLB with 48-h admission, which is often the case in our region.
A series of limitations must be considered in order to correctly interpret the results. Firstly, this is a retrospective observational study with a relatively small number of patients from a single center. Secondly, the patients were probably at an early stage of the disease, as evidenced by the respiratory function values. The cryobiopsy yield in patients in more advanced stages of the disease may differ. Thirdly, specimen evaluation by pathologists with experience in lung pathology is probably essential for this diagnostic yield. Finally, the “patchy” nature of most ILDs could mean that more extensive sample analysis could change the diagnosis in some cases. Furthermore, technical difficulties in reaching the upper lobes could also interfere in the diagnosis.
In conclusion, the use of cryobiopsy in the ILD diagnostic algorithm is a viable and safe alternative for obtaining specimens that enable a histopathological diagnosis to be made in many patients. In experienced hands, this technique would rule out SLB with its associated therapeutic implications in a great many cases, and also significantly reduce costs. Although there are still numerous aspects that must be standardized, such as the size of the probe, freezing time, number of biopsies or their location, the results obtained suggest that cryobiopsy should be considered in the diagnostic management of ILDs. Prospective, comparative, randomized, multicenter studies need to be carried out with a large series of well characterized patients and a common, duly standardized protocol and methodology. This would help define the role of this novel modality in the diagnosis of ILDs.
FundingThis study has not received any type of funding.
Authors’ ContributionsDesign and performance of the study: FHG, CL, MS, JR, MJJ, AX, JS, CA. Data analysis: FHG, CL, JS, CA. Contributions to the manuscript: FHG, CL, MS, JR, MJJ, AX, JS, CA.
Conflict of InterestThe authors declare that they have no conflict of interests.
We would like to thank Sara Castillo and Maite Simó for their invaluable help in performing the endoscopic procedures.
Please cite this article as: Hernández-González F, Lucena CM, Ramírez J, Sánchez M, Jimenez MJ, Xaubet A, et al. Utilidad de la criobiopsia en el diagnóstico de la enfermedad pulmonar intersticial difusa: análisis de rentabilidad y coste. Arch Bronconeumol. 2015;51:261-267.