The GOLD 2011 revision proposes to stratify patients with chronic obstructive pulmonary disease (COPD) by measuring the impact of the disease using the modified Medical Research Council (mMRC) scale or COPD assessment test (CAT). Our aim was to determine whether both methods are equivalent.
Patients and methodsObservational study on a cohort of 283 patients diagnosed with COPD. We analyzed the demographic and lung function results. Patients were assessed by CAT and mMRC on the same day by the same interviewer, and divided into GOLD 2011 categories according to the result of the evaluation. The degree of concordance and Spearman correlation were determined. We used ANOVA on the clinical and functional variables of the four GOLD 2011 categories.
ResultsOn assessing the classification of patients according to the method used, an overall correlation of ρ=0.613 and a degree of concordance of κ=0.63 (moderate) were obtained. κ=0.44 was obtained for the 152 patients in categories A and B (moderate-low), and at 0.38 for the 131 patients in categories C and D (low). Differences were observed between the categories in terms of functional parameters.
ConclusionsThe classification of patients with COPD using the assessment proposed by GOLD 2011 varies according to the method used (CAT or mMRC); more than 25% of patients were reclassified into different categories, implying differences in the recommended therapeutic strategy. Longitudinal studies are needed to appraise which method does better classification of the patients, according to its prognostic ability.
La revisión GOLD 2011 propone estratificar a los pacientes con EPOC midiendo la repercusión de la enfermedad mediante la escala mMRC o mediante el cuestionario CAT. Nuestro objetivo es conocer si la elección de un método u otro resulta equivalente.
Pacientes y métodosEstudio observacional sobre una cohorte de 283 pacientes diagnosticados de EPOC. Se analizaron resultados demográficos, funcionales respiratorios y de evaluación mediante CAT y mMRC, aplicados el mismo día y por el mismo entrevistador a cada paciente. Se distribuyeron en categorías GOLD 2011 según el resultado de la evaluación y se determinó el grado de concordancia y la correlación de Spearman. Se utilizó el test de ANOVA sobre las variables clínicas y funcionales de las 4 categorías GOLD 2011.
ResultadosAl evaluar la clasificación de pacientes según el método empleado, se obtuvo una correlación global ρ=0,613 y un grado de concordancia κ=0,63 (moderado). Se obtuvo κ=0,44 para los 152 pacientes de las categorías A y B (moderado-débil), y de 0,38 para los 131 pacientes de las categorías C y D (débil). Se apreciaron diferencias entre categorías en cuanto a parámetros funcionales.
ConclusionesLa clasificación de los pacientes con EPOC según la evaluación propuesta por GOLD 2011 varía según se emplee CAT o mMRC; se reclasifica a más del 25% de pacientes en diferentes categorías, implicando diferencias en la estrategia terapéutica recomendada. Son necesarios estudios longitudinales que permitan valorar qué método clasifica mejor a los pacientes, atendiendo a su capacidad pronóstica.
Chronic obstructive pulmonary disease (COPD) affects 9.1% of the Spanish adult population aged between 40 and 69 years and is one of the leading causes of death from non-communicable diseases worldwide.1,2 COPD management guidelines specify that diagnosis must include spirometry. This test classifies disease severity according to the percentage of FEV1 compared to the predicted value (FEV1%), and the treatment strategy is defined accordingly.3,4 However, this procedure correlates relatively poorly with patient mortality and symptomatology.5 A multidimensional evaluation that incorporates not only pulmonary function but also quantification of symptoms (e.g. dyspnea scales), the patient's nutritional status and exercise capacity, such as the BODE index,6 has contributed to a change in how this disease is perceived.7
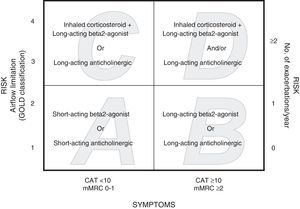
The Global Initiative for Chronic Obstructive Lung Disease (GOLD 2011) revision proposes that patients are stratified according to disease severity, with the incorporation of symptoms determined with the modified Medical Research Council (mMRC) scale or the state of health using the COPD Assessment Test (CAT), as well as the patient's history of exacerbations and post-bronchodilator (pb) FEV1%.8 Patients are classified according to risk: low risk (pbFEV1%≥50% or <2 exacerbations in the previous year) and high risk (pbFEV1%<50% or ≥2 exacerbations in the previous year). The risk index must be determined according to air flow limitation and history of exacerbations. Depending on the symptomatic impact, patients are classified as having less symptoms (CAT<10 or mMRC 0–1) or more symptoms (CAT≥10 or mMRC≥2). Thus, four categories are identified: A (low risk, less symptoms), B (low risk, more symptoms), C (high risk, less symptoms), D (high risk, more symptoms). The proposed therapeutic approach is different for each group8 (Fig. 1).
The aim of this study was to determine from an observational study cohort (BODE Center, Zaragoza, Spain) if the choice of a symptoms scale (mMRC) or a questionnaire measuring quality of life (CAT) originates differences in the assignation to the various severity categories and the corresponding therapeutic implications.
Patients and MethodsStudy DesignThe BODE Project is a multicenter observational study to evaluate the natural history of COPD. Patients were initially selected between 1996 and 2000 and have been followed up on an annual basis. The protocol and major health outcomes have been described previously.6 Diagnosis of COPD was established according to the GOLD criteria8: pbFEV1%/FVC%<0.7, along with an accumulated tobacco use of >20 pack-years. Between January 2010 and September 2012, 283 new patients were evaluated in our center, and this latter study group was used for this analysis.
ProceduresStandardized measurements in the BODE cohort included demographic data, clinical records and health and quality of life questionnaires, including the CAT and mMRC. Both assessments were administered on the same day and by the same interviewer for each patient.
The CAT questionnaire is a self-administered tool for evaluating the quality of life of the COPD patient. It consists of 8 items, producing a score of 0–40; a higher score indicates a poorer state of health.9 The mMRC dyspnea scale is an instrument that establishes dyspnea severity in relation to various physical tasks. It consists of 5 items and is scored on a range of 0 (no dyspnea or only with strenuous exertion) to 4 (dyspnea at rest).10
Spirometry was performed before and 20–30min after inhalation of 200μg salbutamol according to a standardized procedure.11 All patients performed a 6-min walking test.
Assignation of patients to the GOLD 2011 categories was established independently, using the mMRC scale (dyspnea) and the CAT scale (quality of life). The stratification rules specify that first the patient has to be stratified to groups A or C when mMRC is 0–1 or CAT score is <10, or to groups B or D when mMRC is ≥2 or CAT score is ≥10 (Fig. 1). Then the patient's risk has to be established using one of the two procedures. With one procedure, patients with pbFEV1%≥50% are assigned to categories A or B (low risk) and patients with pbFEV1<50% are assigned to categories C or D (high risk). The other procedure consists of establishing the risk on the basis of exacerbations suffered by the patient during the year prior to the evaluation: patients with 0 or 1 exacerbation are stratified to groups A or B (low risk) and patients with ≥2 exacerbations are stratified to groups C or D (high risk). Exacerbations are defined as acute events characterized as a worsening of respiratory symptoms beyond normal day-to-day variations leading to a change in habitual medication.8 An exacerbation was defined as severe when the patient was seen in the emergency room or admitted to hospital. Data from previous exacerbations were obtained from questionnaires administered to the patients and from the databases of the intranet system of the Aragon Healthcare Service. To establish risk, the higher index between airflow limitation measured by pbFEV1% and exacerbation history was selected.
Statistical AnalysisA descriptive analysis was made of the characteristics of the patients included in the series. Data on demographic and clinical variables, respiratory function tests and stress tests were analyzed. The patient cohort was classified into four categories, according to the GOLD 2011 criteria (A, B, C and D), using the CTA questionnaire, and, alternatively, the mMRC scale. The degree of concordance between the results obtained from the application of both methods to the same patient cohort was determined by calculating the weighted kappa (κ) index (agreement or concordance between ordinal qualitative diagnostic tests). The Spearman correlation (non-parametric estimator rho [ρ]) was calculated to evaluate the consistency between the two evaluation methods recorded in the ordinal qualitative scale. The ANOVA test was used to compare the means of the clinical and functional variables in the four GOLD 2011 categories, according to whether one or the other evaluation method was used for classifying the patients. Analyses were performed using SPSS 19.0® software.
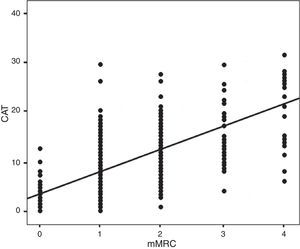
ResultsOf the 283 patients enrolled in the study, 260 (92%) were men and 23 (8%) were women. The mean age was 71±12 years. Main demographic, clinical and functional variables are shown in Table 1. Table 2 shows these variables grouped by sex with an evaluation of any significant differences between the various parameters analyzed. In this visit, 70 (24.7%) patients were still active smokers. The mean pbFEV1% was 62.4±19.9%. Fig. 2 shows the distribution of patients according to their CAT and mMRC values; correlation was calculated with the Spearman coefficient ρ=0.613.
Demographic, Clinical and Functional Variables (Mean±Standard Deviation).
| Total | 283 patients |
| Sex | 260 (92%) males; 23 (8%) females |
| Age | 71±12 years |
| BMI | 28.5±4.8 |
| Active smoker | 70 patients (24.7%) |
| PYI | 61.8±36.77 |
| Comorbidities | HT: 152 patients (53.7%); dyslipidemia: 106 (37.5%); diabetes: 53 (18.7%); cardiovascular episodes: 124 (43.8%); no comorbidity: 48 (17%) |
| pbFEV1% | 62.4%±20.3 |
| PBD(+) | 33 patients (11.6%) |
| Baseline SatO2 | 93.9%±6.3 |
| Walking test | 389.2m±96.2 |
| Spirometry results | |
| FVC | 2.8l±0.8 |
| FVC% | 76.8%±19.8 |
| FEV1 | 1.6l±0.6 |
| FEV1% | 57.5%±19.9 |
| FEV1/FVC | 0.54±0.12 |
| FEV1/FVC% | 0.74±0.17 |
| pbFVC | 3.07l±0.86 |
| pbFVC% | 82.8%±19.25 |
| pbFEV1 | 1.7l±0.7 |
| pbFEV1% | 62.4%±20.3 |
| pbFEV1/FVC | 0.55±0.13 |
| pbFEV1/FVC % | 0.75±17.8 |
FEV1%: forced expiratory volume in one second, percentage of predicted value; FVC: forced vital capacity; HT: hypertension; BMI: body mass index; PYI: pack-year index; pb: post-bronchodilator; PBD(+): positive bronchodilator test; SatO2: oxygen saturation.
Demographic, Clinical and Functional Variables by Sex (Mean±Standard Deviation).
| Males | Females | P | |
| n | 260 (92%) | 23 (8%) | |
| Age | 71.3 years±7.5 | 64.5 years±8.6 | NS (P=.3) |
| BMI | 28.6±4.7 | 27.3±7.1 | P<.01 |
| Active smoker | 60 (23.1%) | 10 (43.5%) | P<.03 |
| PYI | 62.6±33.5 | 53.3±21 | P<.02 |
| pbFEV1% | 62.9%±20.1 | 57.5%±21.8 | NS (P<.6) |
| PBD(+) | 29 (11.1%) | 4 (17.4%) | NS (P=.36) |
| Baseline SatO2 | 93.3%±6.5 | 93.4%±2.7 | NS (P<.5) |
| Walking test | 390.1m±95.1 | 380.3m±111 | NS (P<.4) |
FEV1%: forced expiratory volume in one second, percentage of predicted value; BMI: body mass index; PYI: pack-year index; NS: not significant; pb: post-bronchodilator; PBD(+): positive bronchodilator test; SatO2: oxygen saturation.
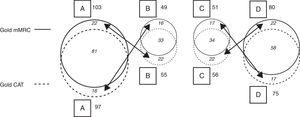
When the CAT questionnaire was used for classifying patients according to the GOLD 2011 criteria, the percentage distribution of patients to the groups was the following: category A, 34.3%; category B, 19.4%; category C, 19.8%, and category D, 26.5%. When the mMRC was applied, the result was: category A, 36.4%; category B, 17.3%; category C, 18.0%, and category D, 28.3% (Table 3 and Fig. 3).
Cohort GOLD 2011 Classification With CAT and mMRC (Fig. 3).
| GOLD CAT | GOLD mMRC | ||||
| A | B | C | D | Total | |
| A | 81 | 16 | 0 | 0 | 97 (35%) |
| B | 22 | 33 | 0 | 0 | 55 (19%) |
| C | 0 | 0 | 34 | 22 | 56 (20%) |
| D | 0 | 0 | 17 | 58 | 75 (26%) |
| Total | 103 (36%) | 49 (17%) | 51 (18%) | 80 (29%) | 283 (100%) |
Explanatory note: When the mMRC scale was applied, 103 patients were classified as A; of these, 22 were classified as B when the CAT questionnaire was applied. Similarly, when the CAT was applied, 97 patients were classified as A, but 16 of these were then classified as B using the mMRC. With the mMRC, 49 patients were classified as B, but 16 of these had been classified as A using the CAT; with the CAT, 55 patients were classified as B, but 22 of these had been classified as A with the mMRC. The same situation arose for categories C and D, depending on whether one questionnaire or the other was applied. There was no migration between AB and CD, as this depends on the risk criteria (AB: low risk; CD: high risk), as can be seen in Fig. 1.
Schematic of migration between GOLD categories depending on results of the application of CAT or mMRC (see distribution of data and explanatory note in Table 3).
When the results from the application of both evaluation methods were examined, it was found that in category A, the classification of 81 patients coincided (83.5% of those classified using CAT and 78.6% of those classified with mMRC). In category B, the classification of 33 patients coincided (60% of those classified with CAT and 67.34% of those classified with mMRC). In category C, the classification of 34 patients coincided (60.7% of those classified with CAT and 66.7% of those classified with mMRC). In category D, the classification of 58 patients coincided (77.3% of those classified with CAT and 72.5% of those classified with mMRC) (Table 3 and Fig. 3).
The κ coefficient for the classification of the patients using the 2 methods was 0.63, indicating a moderate degree of concordance. The calculated κ index was 0.44 for the 152 patients assigned to categories A and B (moderate-weak degree of concordance). The index for the 131 patients classified in categories C and D was 0.38, indicating an even poorer degree of concordance (weak). This implies that for low-risk categories (A and B), the concordance obtained was 75%, while for the high-risk categories (C and D), the concordance was 70%.
The clinical and functional characteristics of the patients in each of the GOLD 2011 categories, depending on the method applied (CAT or mMRC), are shown in Table 4. There were no differences in the categories for age and body mass index (BMI) between patients classified by mMRC or CTA. Only the pack-year index (PYI) was significantly different between category D and the other categories, regardless of whether the classification was made by mMRC or CAT (P<.001). However, the walking test was different between the patients from groups A (fewer symptoms) and B (more symptoms) when classification was performed with mMRC (P<.01), while it was similar between these groups when they were classified using CAT. There were also much greater differences between groups C and D (both with high risk of exacerbations but fewer or more symptoms, respectively) in the walking test when the patients were classified with mMRC (P<.001) vs CAT (P=.003).
Main Clinical and Functional Characteristics of Patients Assigned to GOLD 2011 Categories, as Classified Using mMRC or CAT.
| mMRC | A | B | C | D | CAT | A | B | C | D |
| Age | 70.3 | 70.5 | 69.1 | 72.3 | Age | 71.3 | 68.8 | 70.1 | 71.8 |
| BMI | 28.6 | 29.1 | 28.1 | 28.2 | BMI | 28.5 | 29.3 | 28.1 | 28.2 |
| PYI | 58.4 | 62.3 | 55.7 | 69.7a | PYI | 58.35 | 61.96 | 55.40 | 70.86a |
| pb FEV1% | 75.3 | 69.1 | 56.3 | 44.5 | pb FEV1% | 73.4 | 73.1 | 54.6 | 45.2 |
| Walking test | 414 | 364b | 417 | 344c | Walking test | 400 | 396 | 400 | 354d |
Values expressed as means for each category.
The recent revision of the GOLD 2011 guidelines proposes that when classifying COPD patients, the medical community should take into consideration not only functional deterioration, but also symptoms and risk of exacerbations.8 To establish the degree of functional deterioration, it has been proposed that the same severity grades as those currently used be maintained, i.e., mild (pbFEV1%>80%), moderate (pbFEV1%≥80% and <50%), severe (pbFEV1%≥30% and <50%) and very severe (pbFEV1% <30%). The risk of exacerbation is determined by history of exacerbations in the year prior to the evaluation (<2 or ≥2, respectively). The guidelines leave the choice of method for determining symptoms up to the physician: either the mMRC modified dyspnea scale (few symptoms when mMRC is <2 and more symptoms when mMRC is ≥2) or the CAT quality of life questionnaire (low impact for CAT<10 and high impact for CAT≥10). Our results suggest that the use of one or the other evaluation method changes the assignation of a significant number of patients to one or another category of COPD severity according to the new GOLD guidelines. Thus, these evaluation methods cannot be taken as equivalent, as different treatment strategies are recommended for an individual patient, depending on the use of one or the other method.
The CAT questionnaire reflects the effects of the disease on the patient's health. It is a specific quality of life questionnaire for COPD5,9,12,13 that evaluates not only dyspnea but also other respiratory symptoms, such as the presence and intensity of cough or sputum; it also has a total of 8 items, including exercise capacity, sleep and activities of daily life, producing a score of between 0 and 40. The CAT is a simple questionnaire and may be of use in standard clinical practice. It has proven to be valid for discriminating patient severity.9,12,14,15 However, it is not a diagnostic tool; its purpose is to complement information obtained from pulmonary function measurements and very little is known about its potential prognostic role.14 This questionnaire is particularly sensitive to the effect of changes in health status after exacerbations16 and the effect of respiratory rehabilitation.17 A recent review confirmed the validity of the CAT (among other specific questionnaires) as an instrument for measuring quality of life in COPD, when particularly compared to other generic tools. Unfortunately, this review did not analyze the validity of the role of the mMRC.5 The mMRC is a dyspnea scale that is easier to use, particularly in primary care,18 and can be incorporated into multidimensional tools for the evaluation of COPD, such as BODE6 and ADO (age, dyspnea, obstruction).19 In addition, dyspnea correlates better with the quality of life of COPD patients than objective functional parameters.10,15 Clinical determination of dyspnea reveals the functional capacity of the patient and provides a measurement of the efficacy of the treatment,20 while correlating closely with 5-year survival in patients with COPD.21 It has also shown greater predictive capacity regarding the outcome of respiratory rehabilitation, irrespective of the degree of obstruction.6,18,21
There were no significant differences in age, BMI and FEV1 among patients in the A and B and C and D groups, whether they were classified with mMRC or CAT. However, the exercise capacity of patients in the B vs A and D vs C groups is lower when they are categorized using mMRC rather than CAT. This suggests that dyspnea is much more important than quality of life as a variable affecting the daily life of COPD patients.
COPD patients included in our cohort have similar demographic, clinical and functional characteristics to those from other studies published in the literature, and the distribution of patients over the different categories is also similar.22 In our series, the correlation obtained between the use of the CAT or the mMRC was moderate (ρ=0.613) and similar to that of another cross-sectional cohort study.13 According to our results, the application of either CAT or mMRC for classifying patients to the new GOLD 2011 categories would mean a reclassification of patients in the low (A and B) and high-risk categories (C and D). The calculation of the concordance index depending on whether CAT or mMRC is applied is moderate-weak for the A-B categories (κ=0.44), and even weaker for the C-D categories (κ=0.38). In relative terms, this translates to a concordance of 75% in categories A and B and 70% in categories C and D. Thus, the use of these two evaluation methods is not equivalent, and more than 25% of patients are reclassified after use of one or the other method. This result impacts considerably on the treatment strategies for reclassified patients. The greatest differences in treatment are found among the categories A (the first-line drug treatment recommended is based on short-acting bronchodilators) and B (the first-line drug treatment recommended is based on long-acting bronchodilators and pulmonary rehabilitation). There were small differences between categories C and D, affecting only the alternative drug treatment options, while the recommendations for non-drug treatment are the same.8
A recent study that also compared the outcome of applying both evaluation methods similarly concluded that the new GOLD 2011 classification may need some adjustment. These authors propose a new cut-off point for the application of the mMRC scale (few symptoms=0; more symptoms≥1).23 Two more studies, also recent, carried out in South Korea and in the United States, in very different geographical and sociocultural environments that are unlike Spain, obtained results identical to our own, and drew similar conclusions. One of these studies analyzed 257 South Korean patients in a single center, and obtained results equivalent to our own.24 The other study was a multicenter study that included 4484 North American subjects with COPD, as part of the COPDGene study. They analyzed the results of applying mMRC or the SGRQ questionnaire (as a surrogate for CAT) and concluded similarly that the choice of one or the other tool for measuring symptoms influences the assignation of categories.25
In the latest GOLD 2013 revision, the evaluation of COPD patients has been augmented with the inclusion of the Clinical COPD Questionnaire (CCQ).26 In the initial section of the revision, Methodology and Summary of New Recommendations, and further on in the section Symptom Evaluation, it is made clear that this is a self-administered questionnaire especially designed for measuring the clinical monitoring of COPD patients. The revision underlines that the data support the validity, reliability and sensitivity of this short and easily administered questionnaire. It specifies that on the basis of current knowledge, the cut-off point of CCQ=0–1 may be considered for classifying patients to groups A and C, and a result of CCQ>1 may be used to classify patients to groups B or D. However, the authors declare that more studies are required to validate the discriminative capacity and the practical implications of the CCQ in the detection of exacerbations in daily care. And then, subsequently, in the section on Combined COPD Evaluation, the same proposal for combined evaluation put forward in the GOLD 2011 guidelines, based only on CAT or mMRC for the symptomatic evaluation of the degree of COPD involvement, is maintained, with no explicit mention of the CCQ26
The study presented here has certain limitations: the sample size is relatively small, although the majority of the studies on COPD measurement instruments have a similar or smaller number of patients.5,24 Furthermore, as this is not a cross-sectional study, the prognostic value of the results was not recorded. Nor was any correlation with comorbidities established between the different categories. Comorbidities presented by the patients may have a significant effect on disease prognosis, and should also be taken into account for the combined evaluation of COPD in any future revision of the GOLD guidelines.27
ConclusionsClassification of COPD patients into categories according to the combined evaluation proposed by the GOLD 2011 revision varies according to the evaluation method used in the symptomatic evaluation of patients (CAT or mMRC). More than 25% of patients in the different categories are reclassified if one or the other method is used, implying differences in the recommended treatment strategy. Longitudinal studies are needed to evaluate which assessment method best classifies the patient, with attention being paid to the prognostic capacity of such instruments.
Conflict of InterestsThe authors declare that they have no conflict of interest in the preparation of this manuscript.
Please cite this article as: Rieger-Reyes C, García-Tirado FJ, Rubio-Galán FJ, Marín-Trigo JM. Clasificación de la gravedad de la enfermedad pulmonar obstructiva crónica según la nueva guía Iniciativa Global para la Enfermedad Obstructiva Crónica 2011: COPD Assessment Test versus modified Medical Research Council. Arch Bronconeumol. 2014;50:129–134.