To report a series of stenting procedures for the treatment of malignant superior vena cava (SVC) syndrome.
Material and methodsA review conducted from October 2005 to July 2013 retrieved 56 consecutive patients treated for symptomatic malignant SVC syndrome with stenting.
ResultsSVC stenting was attempted in 56 patients (46 males, 10 females), aged 34–84 years (mean 59.3). The success rate was 49/57 (86%). Success was associated with the type of obstruction and was classified as follows: group 1 (a—SVC stenosis, or b—unilateral innominate vein occlusion with contralateral innominate vein stenosis and normal SVC), group 2 (SVC occlusion excluding bilateral innominate vein occlusion) and group 3 (bilateral innominate vein occlusion irrespective of SVC status). Success rates were 100% (39/39), 75% (9/12) and 16.6% (1/6), respectively. These differences were significant for group 1 versus group 2+3 (P<.001) and for group 2 versus group 3 (P=.032). Acute complications occurred in 9 patients. Patients in whom acute complications occurred were older than the others (67.8 vs 57.6 years, P=.019). Procedure-related death rate was 3.5% (n=2). Stent occlusion occurred in 3.5% (n=2). Patient survival was poor (median 2.6 months; range <1–29.6 months), independent of the success of stenting.
ConclusionsStenting for malignant SVC syndrome provides immediate and sustained symptomatic relief that lasts until death in this set of patients with a short life expectancy and restores the central venous access for administration of chemotherapy. Technical failure was associated with SVC occlusions and primarily with bilateral innominate vein occlusion.
Presentar una serie de intervenciones de implantación de endoprótesis para tratar el síndrome de vena cava superior (VCS) maligno.
Material y métodosEn una revisión del periodo comprendido entre octubre de 2005 y julio de 2013 se identificaron 56 pacientes consecutivos tratados por un síndrome de VCS maligno sintomático mediante implantación de endoprótesis.
ResultadosLa implantación de endoprótesis en la VCS se intentó en 56 pacientes (46 varones, 10 mujeres) de 34-84años de edad (media 59,3). La tasa de éxitos fue de 49/57 (86%). El éxito se asoció al tipo de obstrucción agrupada de la siguiente forma: grupo 1 (a: estenosis de VCS, o b: oclusión de vena innominada unilateral con estenosis de vena innominada contralateral y VCS normal), grupo 2 (oclusión de VCS y exclusión de oclusión de vena innominada bilateral) y grupo 3 (oclusión de vena innominada bilateral con independencia del estado de la VCS). Las tasas de éxito fueron del 100% (39/39), del 75% (9/12) y del 16,6% (1/6), respectivamente. Estas diferencias eran significativas: grupo 1 frente a grupo 2+3 (p<0,001) y grupo 2 frente a grupo3 (p=0,032). Se produjeron complicaciones agudas en 9 pacientes. Los pacientes en los que se dieron las complicaciones agudas fueron de mayor edad que los demás (67,8 frente a 57,6años, p=0,019). Hubo muertes relacionadas con la intervención en el 3,5% (n=2). Se produjo una oclusión de la endoprótesis en el 3,5% (n=2). La supervivencia de los pacientes fue baja (mediana 2,6; rango <1-29,6meses) e independiente del éxito de la implantación de endoprótesis.
ConclusionesLa implantación de endoprótesis para el síndrome de VCS maligno proporciona un alivio sintomático inmediato y sostenido que persiste hasta la muerte en este grupo de pacientes con una esperanza de vida corta y restablece el acceso venoso central para la administración de quimioterapia. El fallo técnico se asoció a oclusiones de la VCS y sobre todo a una oclusión de la vena innominada bilateral.
Superior vena cava (SVC) syndrome is caused by an obstruction in the veins returning from the head, neck and upper extremities to the right atrium of the heart. The obstruction may occur in the SVC or in both innominate veins. Around 95% of the cases are caused by malignant tumors and the remaining 5% are due to benign disorders. Malignant SVC syndrome occurs in 3%–5% of patients with advanced intrathoracic malignancies.1 The obstruction of venous drainage is a consequence of the SVC being compressed by a tumor in the right primary bronchus or the right upper lobe, or by a large mediastinal lymphadenopathy of the right precarinal or paratracheal lymph nodes.2 This may lead to secondary venous thrombosis. SVC invasion is rare. Clinical manifestations consist of edema of the face, the periorbital and cervical regions and both upper extremities, dilation of superficial veins and facial redness, dyspnea, cough, snoring, dysphagia, headache, blurred vision and cognitive changes. It may lead to death and coma resulting from cerebral edema or airway obstruction due to glottal or bronchial edema. Severity depends on the degree of obstruction and the speed of onset. Treatment is palliative. Medical treatment includes the use of diuretics, corticosteroids and anticoagulants. Chemotherapy and radiotherapy take about 3 weeks to become effective and have significant side effects. The success rate of these procedures is 77% in small cell lung cancer (SCLC) and 60% in non-small cell lung cancer (NSCLC), with recurrence rates of 16.5% and 11%, respectively.3 Stenting procedures compare favorably with these approaches, since they provide risk-free relief in less than 72h, with a success rate of 95%. The recurrence rate is 11% but this can be treated with re-intervention. Long-term patency is 92%.3 Surgical treatment is not an option in patients with short life expectancy and poor general condition.
Materials and MethodsA review was carried out between October 2005 and July 2013 (retrospectively until October 2008 and prospectively from then on). Fifty-six (56) consecutive patients were identified who had received treatment for symptomatic malignant SVC syndrome with 57 SVC stent placements. SVC stenting was the first line of treatment offered to all patients with malignant SVC, regardless of the available tumor histology or the current or foreseeable use of chemotherapy or radiotherapy. Patients were excluded if they could not remain in decubitus or semi-decubitus position (n=2), if they had asymptomatic SVC detected on computed tomography (CT) (n=3), and if they had SVC of benign origin (n=2). Clinical diagnosis was confirmed by CT. An anteroposterior chest X-ray was performed the day after stenting to confirm positioning and expansion. A CT was performed if clinically indicated. Data were retrieved from clinical records and the National Death Registry. None of the patients were lost to follow-up.
Statistical AnalysisVariables associated with the success rate (SR) and complications were analyzed using the Fisher exact test for categorical variables and the t-test for continuous variables. A multivariate logistic regression analysis was used. The confidence intervals (CI) for the SR values were calculated using a binomial distribution model. Survival was estimated using the Kaplan–Meier method and an analysis of related variables was performed using the log-rank test. A P-value <.05 was considered statistically significant. The statistical analysis was performed using SPSS Statistics 20.0 software.
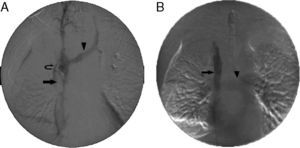
ProcedureSVC stenting was performed in the operating room with a portable digital imaging system (Philips, BV Endura). The SVC obstruction was generally approached via a femoral vein access using a PTFE-coated guidewire with a J-tip or a standard hydrophilic angled 0.035-inch guidewire, with the support of a vertebral catheter or multi-purpose 5F Berenstein catheter oriented with anatomical reference points. An initial digital subtraction venogram was performed after passing the obstruction, via the catheter situated in a superior location in the innominate or jugular vein. An intravenous bolus of heparin 5000IU was then injected. For very narrow lesions, balloon dilation was employed before stent placement. Stent size was determined from the CT and venogram during the procedure. Obstruction in both innominate veins was treated with unilateral stent placement, as described elsewhere.4 The side for recanalization was the side with a patent jugular vein. If both jugular veins were patent, the right side was preferred, since its trajectory is straight. Balloon dilation was performed after deployment until a diameter of 12–18mm was achieved. After completion of the procedure, a venogram was performed to evaluate flow and diameter of the SVC and the pulmonary arteries (Fig. 1). The patients were discharged with a prescription for enoxaparin 40mg and acetylsalicylic acid 100mg/day for life. If there was associated thrombosis, enoxaparin was administered at full therapeutic doses for at least 3 months, followed by a dose of 40mg. Patients with bleeding complications did not receive anticoagulation or anti-platelet treatment for a variable period of time.
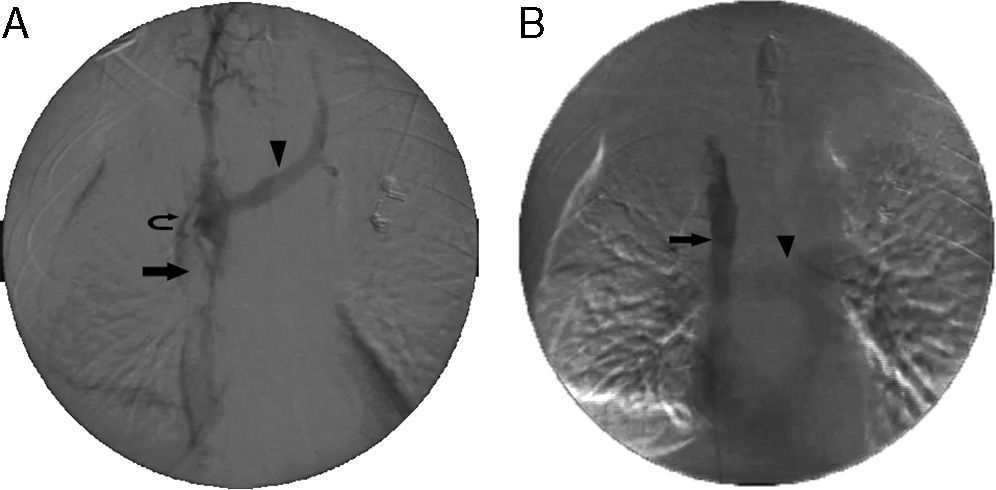
Stent placement in the superior vena cava (SVC). (A) Initial venogram obtained via the femoral access with the catheter tip placed in the right innominate vein, showing SVC occlusion (arrow), retrograde filling of the left innominate vein (arrowhead) and collateral circulation (curved arrow). (B) Final venogram after placement of a 14×60mm stent in the SVC (arrow). The left innominate vein and contralateral circulation can no longer be seen. The increased return of venous blood to the SVC and the right atrium leads to pulmonary artery filling (arrowhead).
SVC stent placement was attempted in 56 patients (46 men and 10 women) aged between 34 and 84 years (mean 59.3; standard deviation [SD] 10.7). Tumor samples were obtained by transbronchial biopsy (n=40, 71.4%), transtracheal biopsy (n=2, 3.6%), mediastinoscopy (n=10, 17.9%), and pulmonary decortication (n=1, 1.8%), or no sample was obtained (n=3, 5.4%). Underlying malignancies were lung adenocarcinoma (n=17, 30.4%), small cell lung carcinoma (n=15, 26.8%), squamous cell lung carcinoma (n=9, 16.1%), large cell neuroendocrine carcinoma (n=2, 3.6%), lymphoma (n=2, 3.6%), mesothelioma (n=1, 1.8%), metastatic breast cancer (n=2, 3.6%), metastatic renal carcinoma (n=1, 1.8%), undifferentiated (n=3, 5.4%) and unknown (n=4, 7.1%). TNM staging was as follows: stage IIIA (n=2, 3.6%), stage IIIB (n=13, 23.2%) and stage IV (n=41, 73.2%). Treatments were pulmonary resection (n=3, 5.4%), chemotherapy (n=24, 42.9%), radiotherapy (n=3, 5.4%), chemotherapy and radiotherapy (n=25, 44.6%) or no adjuvant treatment (n=4, 7.1%). In 27 (48.2%) patients, SVC syndrome was an initial manifestation of the disease, defined as a time between diagnosis and stent placement of less than 30 days (Table 1).
Patient Characteristics.
| Characteristic | Value |
| Number of patients | 56 |
| Number of procedures | 57 |
| Sex, n (%) | |
| Males | 46 (82.1) |
| Females | 10 (17.9) |
| Age in years, mean (SD) | 59.3 (10.7) |
| Biopsy route of approach, n (%) | |
| Bronchoscopy | 40 (71.4) |
| Transtracheal | 2 (3.6) |
| Mediastinoscopy | 10 (17.9) |
| Pulmonary decortication | 1 (1.8) |
| None | 3 (5.4) |
| Cancer histology, n (%) | |
| Adenocarcinoma | 17 (30.4) |
| Small cell carcinoma | 15 (26.8) |
| Squamous cell carcinoma | 9 (16.1) |
| Large cell neuroendocrine carcinoma | 2 (3.6) |
| Lymphoma | 2 (3.6) |
| Mesothelioma | 1 (1.8) |
| Metastatic breast adenocarcinoma | 2 (3.6) |
| Metastatic renal carcinoma | 1 (1.8) |
| Undifferentiated | 3 (5.4) |
| Unknown | 4 (7.1) |
| TNM cancer staging, n (%) | |
| IIIA | 2 (3.6) |
| IIIB | 13 (23.2) |
| IV | 41 (73.2) |
| Pulmonary resection, n (%) | 3 (5.4) |
| Adjuvant treatment, n (%) | |
| Chemotherapy | 24 (42.9) |
| Radiotherapy | 3 (5.4) |
| Chemotherapy and radiotherapy | 25 (44.6) |
| None | 4 (7.1) |
| Time interval between diagnosis and stent placement, n (%) | |
| <30 days | 27 (48.2) |
| ≥30 days | 26 (46.4) |
| Biopsy not performed | 3 (5.4) |
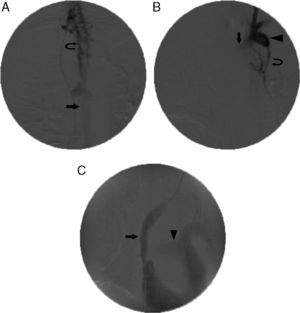
Local anesthesia was used according to the standard practice. General anesthesia was used in 2 patients (3.5%): one non-cooperative schizophrenic patient and one patient undergoing simultaneous mediastinoscopy. The standard percutaneous access was via the right femoral vein. The left femoral vein was used in 3 cases after failure to achieve right femoral access. Right jugular access was used in the first patient only. Left jugular was combined with right femoral access in two cases of bilateral innominate vein occlusion. In one of these cases, the procedure was discontinued after the obstruction was overcome, due to ventricular tachycardia. In the others, it was achieved with the through-and-through guidewire technique (Fig. 2). The location and type of the venous obstruction, and stent placement positioning were determined. The stents used were Sinus-XL (Optimed) (n=20, 30.5%), Smartstent (Cordis) (n=15, 26.3%), Wallstent (Boston Scientific) (n=11, 19.3%) and Express (Boston Scientific) (n=3, 5.9%). The number of stents per patient ranged between 0 and 4. The length of the stent was between 0 and 160mm and stent diameter ranged between 10 and 24mm. The mean duration of the procedure was 54.3min, with an SD of 29min (Table 2).
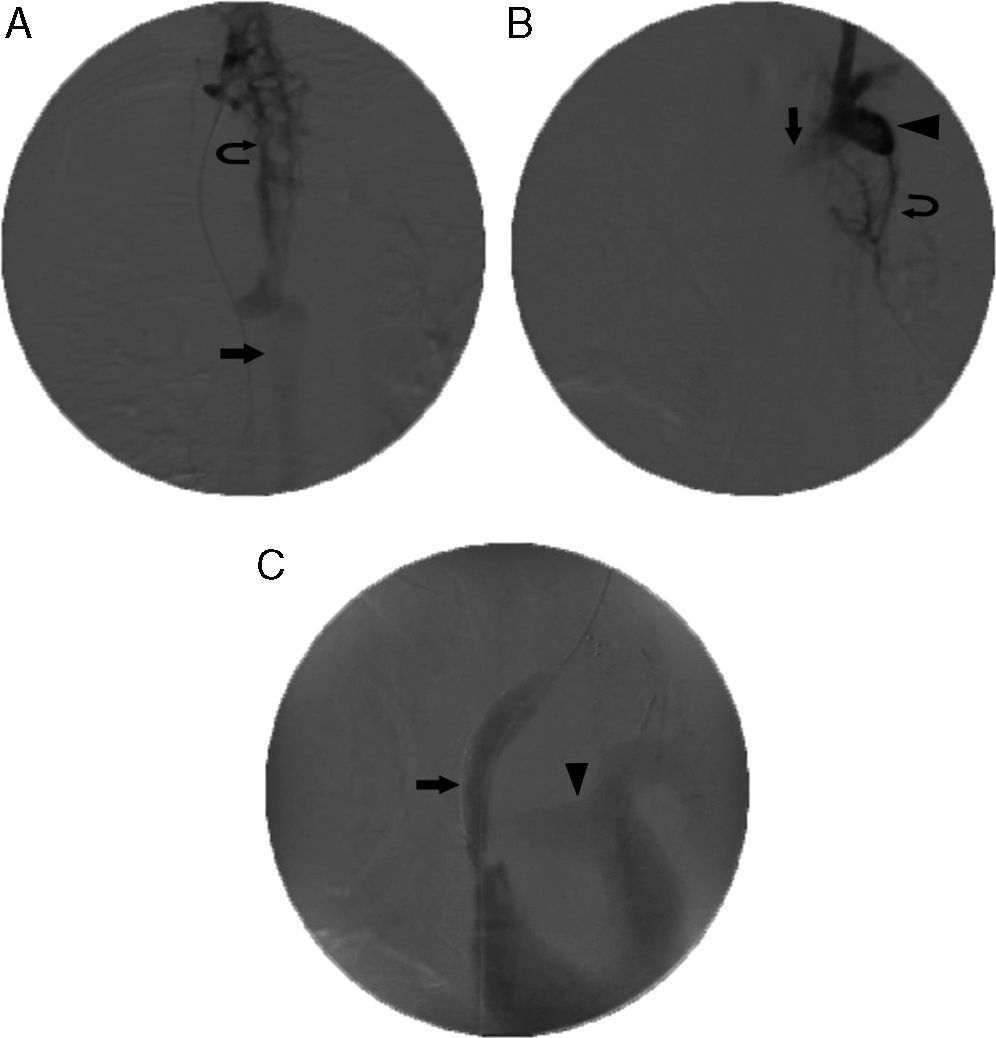
Stent placement in superior vena cava (SVC) and left innominate vein. (A) Initial venogram obtained from the femoral access route with the catheter tip placed in the right internal jugular vein, showing occlusion of the SVC and bilateral innominate veins, widening and retrograde filling of the azygous vein (arrow) and collateral circulation (curved arrow). (B) Venogram obtained with left internal jugular vein access, showing occlusion of the right innominate vein (arrow), retrograde filling of the subclavian vein (arrowhead) and collateral circulation (curved arrow). (C) Final venogram after placement of two overlapping 18×60 and 20×60mm stents in the SVC and the innominate vein (arrow). Energetic filling of the pulmonary artery can be seen (arrowhead). Obstruction was overcome via a left jugular vein access route, guidewire with loop and stents inserted via the right femoral vein (through-and-through technique or flossing).
Stent Placement Characteristics.
| Characteristic | Value |
| Anesthesia, n (%) | |
| General | 2 (3.5) |
| Local | 55 (96.5) |
| Vascular access, n (%) | |
| Right femoral | 51 (89.5) |
| Left femoral | 3 (5.3) |
| Right femoral and left jugular | 1 (1.8) |
| Right femoral and left jugular (through-and-through) | 1 (1.8) |
| Right jugular | 1 (1.8) |
| Location of venous obstruction, n (%) | |
| Right jugular, right innominate vein and left innominate vein | 3 (5.3) |
| Right jugular, right innominate vein, left innominate vein and superior vena cava | 2 (3.5) |
| Right innominate vein and superior vena cava | 3 (5.3) |
| Right innominate vein, left innominate vein and superior vena cava | 6 (10.5) |
| Right innominate vein and superior vena cava | 1 (1.8) |
| Superior vena cava | 42 (73.7) |
| Type of venous obstruction, n (%) | |
| Group 1 | 39 (68.4) |
| (a) Superior vena cava stenosis (SVC) | 37 (64.9) |
| (b) Unilateral innominate vein occlusion with contralateral innominate vein stenosis and normal SVC | 2 (3.5) |
| Group 2 | 12 (21.0) |
| SVC occlusion, excluding bilateral innominate vein occlusion | |
| Group 3 | 6 (10.5) |
| Bilateral innominate vein, irrespective of SVC status | |
| Location of stent placement, n (%) | |
| None | 8 (14.0) |
| Right jugular vein, right innominate vein and superior vena cava | 1 (1.8) |
| Right innominate vein and superior vena cava | 5 (8.8) |
| Left innominate vein and superior vena cava | 6 (10.5) |
| Superior vena cava | 37 (64.9) |
| Type of stent, n (%) | |
| None | 8 (14.0) |
| Sinus-XL (Optimed) | 20 (30.5) |
| Smartstent (Cordis) | 15 (26.3) |
| Wallstent (Boston Scientific) | 11 (19.3) |
| Express (Boston Scientific) | 3 (5.9) |
| Stent by procedure, n (%) | |
| 0 | 8 (14.0) |
| 1 | 35 (61.4) |
| 2 | 9 (15.8) |
| 3 | 3 (5.3) |
| 4 | 2 (3.5) |
| Stent length in mm, n (%) | |
| 0 | 8 (14.0) |
| 40 | 10 (17.5) |
| 60 | 28 (49.1) |
| 80 | 1 (1.8) |
| 100 | 5 (8.8) |
| 120 | 2 (3.5) |
| 160 | 3 (5.3) |
| Maximum stent diameter in mm, n (%) | |
| 0 | 8 (14.0) |
| 10 | 3 (5.3) |
| 12 | 4 (7.0) |
| 14 | 11 (19.3) |
| 16 | 5 (8.8) |
| 18 | 15 (26.3) |
| 20 | 6 (10.5) |
| 22 | 4 (7.0) |
| 24 | 1 (1.8) |
| Duration of procedure in minutes, mean (SD) | 54.3 (29) |
The SR was 49/57 (86%). Success was associated with the type of obstruction, classified as follows: group 1 (a: SVC stenosis and b: unilateral innominate vein occlusion with contralateral innominate vein stenosis and normal SVC), SR: 39/39 (100%), CI: 0.905–1.00; group 2 (SVC occlusion excluding bilateral innominate vein exclusion), SR: 9/12 (75%), CI: 0.35–0.797; and group 3 (bilateral innominate vein occlusion irrespective of SVC status), SR: 1/6 (16.6%), CI: 0.004–0.64 (Table 3). These differences were statistically significant for group 1 versus group 2+3 (P<.001) and for group 2 versus group 3 (P=.032). The bivariate analysis showed a better SR for men (43/47 versus 6/10, P=.025) and when the biopsy was transbronchial compared to mediastinoscopy, transtracheal puncture or pulmonary decortication (37/40 versus 4/14, P=.065). The multivariate analysis showed that only the type of obstruction was related to SR: group 1 versus group 2+3 (P=.003; odds ratio: 0.037). SR was unrelated to age, cancer histology, TNM staging, adjuvant treatments and procedure duration. All patients in whom the procedure was successful had total or partial relief of SVC syndrome symptoms.
Stent Placement Outcomes.
| Results | Value |
| Success rate, n (%) | 49/57 (86.0) |
| Group 1 | 39/39 (100) |
| (a) Superior vena cava (SVC) stenosis | 37/37 (100) |
| (b) Unilateral innominate vein occlusion with contralateral innominate vein stenosis and normal SVC | 2/2 (100) |
| Group 2 | 9/12 (75.0) |
| SVC occlusion excluding bilateral innominate vein occlusion | |
| Group 3 | 1/6 (16.6) |
| Bilateral innominate vein occlusion, irrespective of SVC status | |
| Acute complications, n (%) | 9/57 (15.8) |
| Stent migration | 3 (5.3) |
| Cardiac dysrhythmia | 3 (5.3) |
| Asystole and bradycardia | 1 (1.8) |
| Supraventricular tachycardia | 1 (1.8) |
| Ventricular tachycardia | 1 (1.8) |
| Bleeding | 3 (5.3) |
| Hemopericardium | 1 (1.8) |
| Hemoptysis | 1 (1.8) |
| Psoas hematoma | 1 (1.8) |
| Procedure-related death, n (%) | 2 (3.5) |
| Stent occlusion, n (%) | 2 (3.5) |
| Asymptomatic | 1 (1.8) |
| Symptomatic | 1 (1.8) |
| Patient survival (months) | |
| Median | 2.7 |
| Range | 0–29.6 |
The rate of acute complications was 15.8% (n=9). Patients who had complications were older than the others (67.8 versus 57.6 years, P=.019). Stent migration toward the right atrium was observed in 3 patients (5.3%): one patient with stent placement in the SVC and 2 with stents placed in the confluence of the left innominate vein (LIV) and the SVC. Migration was halted with the proximal placement of one or two additional stents. Dysrhythmias were observed in 3 patients (5.3%): one had asystole and bradycardia after stenting, requiring transvenous cardiac pacing (n=1, 1.8%); another had ventricular tachycardia during manipulation of the intracardiac guidewire from a left jugular approach, treated with electric cardioversion, leading to discontinuation of the procedure (n=1, 1.8%); and another had supraventricular tachycardia after stent placement in the LIV and SVC, treated with intravenous amidarone (n=1, 1.8%). Three patients (3) developed bleeding (5.3%): hemopericardium in a patient with previous pericardial effusion that expanded but did not collapse the right ventricle−300 cc of blood fluid was obtained by periocardiocentesis (n=1, 1.8%); hemoptysis after stent placement in a patient who had bled previously and who was immediately treated with laser bronchoscopy (n=1, 1.8%); and hematoma of the left psoas, contralateral to the femoral venous access, diagnosed three days after stent placement, that produced a fall in hemoglobin from 10.1 to 5.8mg/dl, which finally resolved (n=1, 1.8%). Stent occlusion occurred in 2 patients (3.5%): one was asymptomatic and occurred 3 months after stent placement due to SVC stenosis that was left untreated; the other was symptomatic, occurring 1.5 months after stent placement and was treated with successful re-stenting of the LIV and the SVC.
Procedure-related death occurred in 2 patients (3.5%). One of these was the first patient in this series, who died 6h after the procedure: no cause could be identified. The other patient had migration of the stents implanted in the LIV and the SVC, and died 8h after the procedure, presumably due to additional stent migration (Table 3).
Patient survival was poor (median 2.7 months, range <1–29.6 months). Six patients survived more than one year. Survival appeared lower in patients with SVC syndrome diagnosed in the 30 days following tumor biopsy (P=.078). Survival was low in the 4 patients who were thought to be in a too advanced stage for chemotherapy or radiotherapy (range 0–0.63 months compared to 0–29.6 months, P<.001). No differences were observed between patients who had successful stent placement and those in whom it failed in terms of survival, procedure-related complications, cancer histology, TNM staging, adjuvant treatments, SVC obstruction type, age or sex (Table 3).
DiscussionSVC stent placement is a rapid and effective treatment for SVC syndrome if the patient can remain in a decubitus position and is not allergic to iodinated contrast materials.
There are several techniques that are worth mentioning. A venogram can be obtained by the jugular access that is useful for guiding the passage through the obstruction. This access point also provides a shorter route to the SVC, but it is more laborious and uncomfortable for the patient and the operator. In addition, the swelling in the neck may make it difficult to puncture, and if the procedure is not successful, hemostasis of the venous access may be problematic, particularly if 10F sheaths are used. To avoid sheath migration, the use of an oversized stent with a length of more than 60mm is required. Single stents are preferable to folded devices, particularly in the confluence of the LIV and the SVC, since the curve makes the stent fixation unstable. In 3 of 6 cases, there was incomplete stent apposition in the upper end along the length of the internal curve of the stents placed in the LIV and SVC confluence (bird-beak configuration), although there was no flow perturbation. To use an oversized stent, the variability of the vein diameter depending on fluid balance, body posture and resolution of the obstruction due to stenting, chemotherapy or radiotherapy must all be taken into account. The thrombus may make the stent fixation surface slippery. Residual stenosis refractory to balloon dilation was often observed, but this was not associated with clinical failure.
Several types of stent have been used for treating SVC syndrome: balloon-expanding, self-expanding stainless steel, self-expanding nitinol and self-expanding PTFE-coated nitinol. Various publications find similar results for the different stents.4–12 Fagedet et al.11 reported more complications with stents with a diameter larger than 16mm. However, the stent diameter is determined by the native vessel itself and an undersized stent cannot be used to overcome this characteristic. Recently, a comparative study of treatment with coated stents compared to that with uncoated stents showed that the former provides greater cumulative patency, although there were no differences in clinical success or patient survival.12
Compared to other series with mixed benign and malignant etiologies5,6 or malignant etiology only,4,7–12 the SR was lower (86% compared to 97.6%). This may be because the stent placement procedure was abandoned in 7 of 8 unsuccessful cases, after the obstruction could not be passed via the femoral access route, and no attempt at jugular or humeral access was made. The SR was 100% in group 1 (stenosis), 75% in group 2 (occlusions) and 16.6% in group 3 (bilateral innominate vein occlusion). Better results have been described for stenosis than for occlusion.8 The acute complication rate was higher in elderly patients, and this coincides with the findings of other reports (15.8% compared to 6.7%–15%).7–11 Cardiac dysrhythmias, stent migration and bleeding complications occurred at the same rate (5.3%). These complications were serious and required specialized procedures for treatment: electrical cardioversion, cardiac pacing, pericardiocentesis and laser bronchoscopy. Procedure-related mortality was similar to that of other series (3.5% versus 0%–2.4%).7–11 Stent occlusion rate was lower (3.5% versus 13.4%–21.9%),7–11 which may be due to the exclusion of the difficult cases in which the procedure failed. These cases could be more liable to subsequent thrombosis,11 inexact follow-up or a shorter patient survival in this series (median 2.7 months versus 6 months). Patients with stent placement success or failure had similar survival, underlining the palliative nature of this procedure in SVC. Patients in whom the SVC appeared as an initial manifestation of their disease had a shorter survival than others, possibly due to the fact that early appearance of SVC is a sign of a more aggressive form of the disease.
ConclusionStent placement is an effective first-line treatment in malignant SVC syndrome. It provides immediate and sustained clinical relief and a central access route for the administration of chemotherapy.
AuthorshipGonçalo Sobrinho conceived the study, performed the interventions, obtained the data and analyzed and interpreted the results and prepared the article.
Pedro Aguiar carried out the data analysis.
Conflict of InterestsThe authors declare that they have no conflict of interests.
The authors thank Dr Paula Wright for reviewing the manuscript.
Please cite this article as: Sobrinho G, Aguiar P. Implantación de endoprótesis para el tratamiento del síndrome de vena cava superior maligno: serie de 56 pacientes de un solo centro. Arch Bronconeumol. 2014;50:135–140.