Despite the high incidence of bronchiolitis among children, post-infective bronchiolitis obliterans (PIBO) is an uncommon pediatric complication. The microorganism most frequently associated with bronchiolitis obliterans is adenovirus, followed by Mycoplasma pneumoniae, parainfluenza virus, influenza virus, etc.1 The obliterative fibroblastic process in the bronchioles causes air trapping and respiratory failure that can improve with time, leaving severe obstructive functional impairment.2 In children older than 12 months, fibroblastic scarring of the bronchial lumen has been described after infectious processes, an entity known as bronchitis obliterans. In this case, occlusion of the lumen can be associated with atelectasis of the pulmonary anatomical region. However, this radiological finding is not exclusive to bronchitis obliterans, and is common in PIBO. Local treatment consists of partial or complete lung resection. When involvement is generalized, the disease course is fatal, unless lung transplantation can be performed.3
We report the case of a 13-year-old girl, with no significant clinical history, who consulted due to a catarrhal syndrome with fever, treated with amoxicillin-clavulanate acid. Twenty-four hours later, she attended the emergency room due to progressive dyspnea. On arrival, the patient was showing signs of severity with raised inflammatory markers, and the chest radiograph showed bilateral alveolar consolidation. Treatment was started with cefotaxime and levofloxacin, and she was admitted to the intensive care unit for monitoring. Her clinical situation and arterial blood gases quickly deteriorated, and intubation was required. Influenza B virus RNA was identified in the nasal swab. Blood cultures and antigen detection in urine for pneumococcus and Legionella were negative. Given her poor response to mechanical ventilation and the possible need for extracorporeal membrane oxygenation (ECMO) support, the patient was transferred to our hospital.
After methicillin-resistant Staphylococcus aureus was isolated from the blood culture and the bronchoalveolar lavage, the cephalosporin was discontinued and treatment continued with clindamycin. After 7 days of intubation, 2 days of non-invasive ventilation, and 17 days of oxygen therapy, the patient was discharged, 28 days after admission, receiving bronchodilation with salbutamol.
Outpatient monitoring confirmed radiological resolution of the infiltrates and persistent retrocardiac segmental atelectasis. The patient had a slight cough, persistent wheezing, and dyspnea on moderate exertion, so the bronchodilator treatment was intensified and treatment began with inhaled corticosteroids. However, limited clinical improvement and severe functional obstructive impairment (FVC 1.9 l (55%); FEV1 0.76 l (26%); MMEF 0.22 l/s (9%); FEV1/FVC 48%) led to the initiation of oral corticosteroid treatment. PIBO was suspected, so a pulmonary CT was performed, revealing tracheobronchomegaly, bilateral diffuse bronchiectasis, and generalized air trapping.
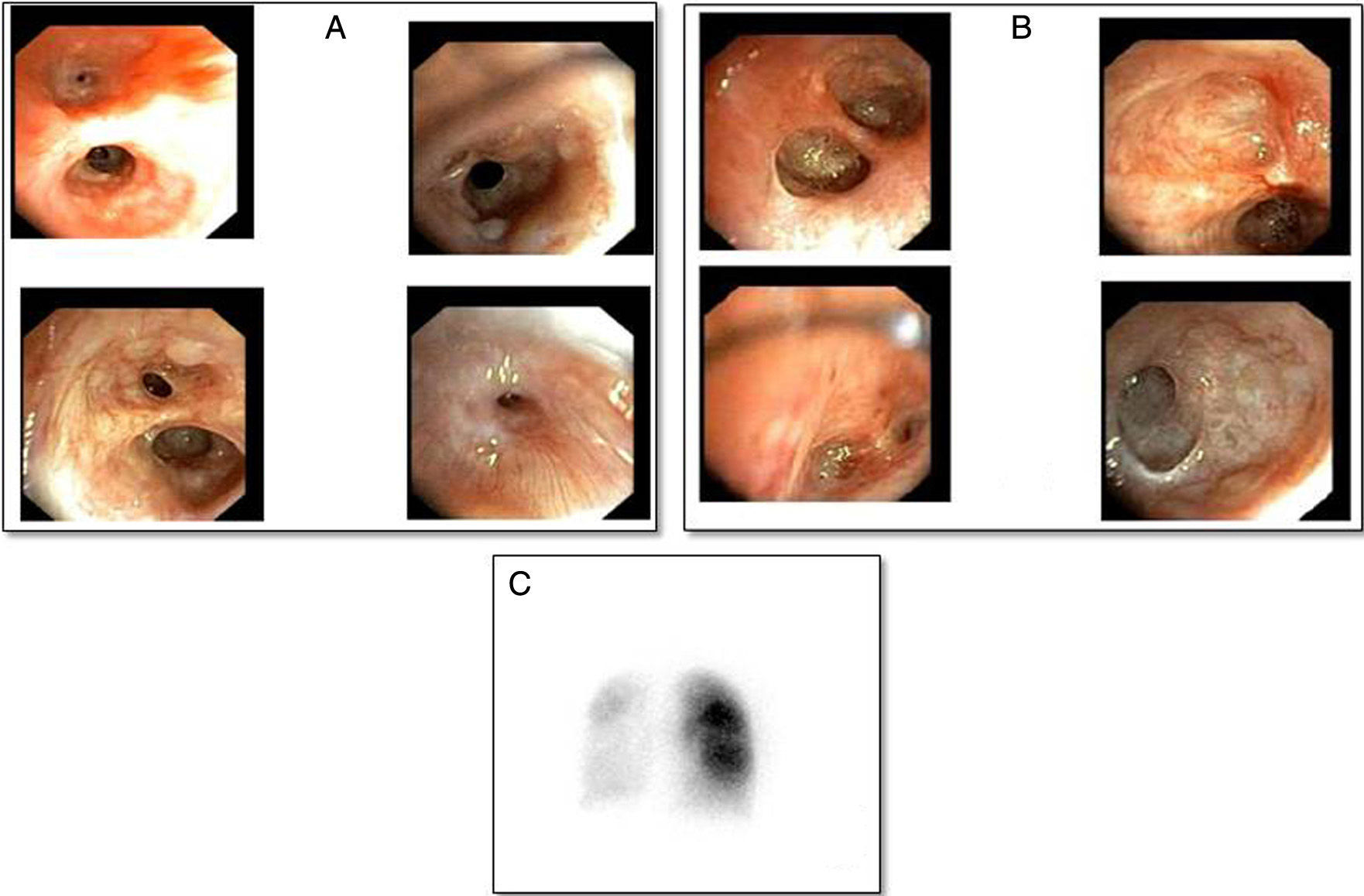
Three months after discharge she developed clinical worsening with a normal chest radiograph, requiring hospitalization. She was given methylprednisolone iv (500mg/day/3 days) and nebulized bronchodilators. After initial improvement, she developed respiratory failure due to atelectasis of the left lung and consequent pneumothorax that did not require a chest tube. A bronchoscopic study was performed, in which the findings shown in Fig. 1 were observed. After the membranes that were occluding the segmental bronchi of the left upper lobe were perforated, the patient showed transient resolution of the atelectasis, and then of the pneumothorax, so perforation of the endobronchial septa was repeated, and the patient was treated with CPAP positive pressure with an oronasal mask. Scintigraphy with quantification of pulmonary perfusion showed hypoperfusion in the left lung and to a lesser extent in the right lower lobe: left lung: 17% (upper 10%, lower 7%), right lung: 83% (upper 51%, lower 32%) (Fig. 1C).
(A) Right bronchus with mucinous cysts and mild stenosis of the segmental and subsegmental bronchi of the right upper lobe, obliteration of the bronchi of the medial segment of the right middle lobe, segment 6 and stenosis of the posterior, anterior, and medial lung bases, obliteration of the lateral lung base. (B) Left bronchus showing scarring obliterating the lumen of the subsegmental subdivisions of the left upper lobe, lingula, and left lower lobe. (C) Lung scintigraphy showing severe overall hypoperfusion of the left lung. Right lung with greater perfusion in the upper lobe than in the lower lobe.
The patient was included in the lung transplantation wait list, and the procedure was performed 6 weeks later. Immediate post-surgical progress was good. Four months after transplantation, she did not require oxygen, and lung function and clinical examination were normal. Histology of the explant showed bronchial and bronchiolar ectasias, patchy bilateral constrictive bronchiolitis with fibroblasts and foamy histiocytes, and adjacent cystic formations caused by dilation of the mucinous glandular component of the airway.
Pulmonary complications associated with influenza virus infection include necrotizing tracheobronchitis, acute respiratory distress syndrome, PIBO, and secondary bacterial pneumonia. Staphylococcus aureus is the most common coinfection in influenza virus pneumonia, and is associated with an increased rate of suppurative complications.4
Initial clinical manifestations of bronchitis obliterans are non-specific: persistent cough, wheezing, tachypnea, dyspnea, and loss of appetite. Although it has been suggested that bronchitis obliterans is a different entity from PIBO,3 the histological study of our patient's lungs showed associated bronchiolar scarring. A diagnosis of PIBO based on spirometric and radiological findings (CT) after a severe pulmonary infection is now generally accepted.1,2,5 Septation caused by bronchial scarring might be diagnosed only in the presence of complications such as lobar or pulmonary atelectasis that require bronchoscopic intervention, as in our case. Systematic bronchoscopic exploration might assist in the early identification of bronchitis obliterans.
Perforation of the scar membranes has been attempted in the few pediatric cases published, but response is short-lived. For this reason, conservative treatment has been suggested.3 However, when involvement is generalized, ventilatory failure may be irreversible, and only manageable with ECMO and lung transplantation. Our patient underwent bronchoscopic intervention to reverse pulmonary atelectasis on 2 occasions: lung expansion could be achieved but normal functionality could not be recovered, as shown on the lung scintigraphy. Generalized involvement led to the patient's inclusion in the lung transplantation wait list, and no bridging ECMO support was required.
The response of PIBO to drug treatment, consisting of high doses of corticosteroids, preferably in monthly boluses, is variable. Although the duration of the inflammatory process has not been defined, it is generally agreed that starting treatment early, before fibrosis occurs, is advisable, and that is should continue for at least 6 months.5
Please cite this article as: Santos ADH, Martínez JE, Román CGS, Andreu JAL. Bronquitis obliterante secundaria a neumonía por virus Influenzae B y sobreinfección por Staphylococcus aureus. Arch Bronconeumol. 2017;53:463–464.