Nontuberculous mycobacteria (NTM) are environmental organisms residing in soil and water. A greater number of isolates of NTM species has led to a higher prevalence of pulmonary infections caused by NTM.1 Over 150 different species of NTM have been described. NTM-induced pulmonary infections are usually caused by Mycobacterium avium-intracellulare, M. kansasii, and M. abscessus.1 NTM are traditionally divided into slowly-growing and rapidly-growing organisms.2M. szulgai is a slow-growing organism that was first reported by Marks et al. in 1972.3 It is isolated from environmental sources, including snails, aquarium water, swimming pool water, and tropical fish. However, this organism is rarely isolated from humans, and accounts for just <0.5% of all human isolates of NTM.4 Pulmonary infection is the most common manifestation of M. szulgai, and clinically and radiologically it resembles the manifestation of M. tuberculosis and M. kansasii.2 Here we describe two cases of pulmonary M. szulgai infection: 1 with pneumothorax and the other with spontaneous remission.
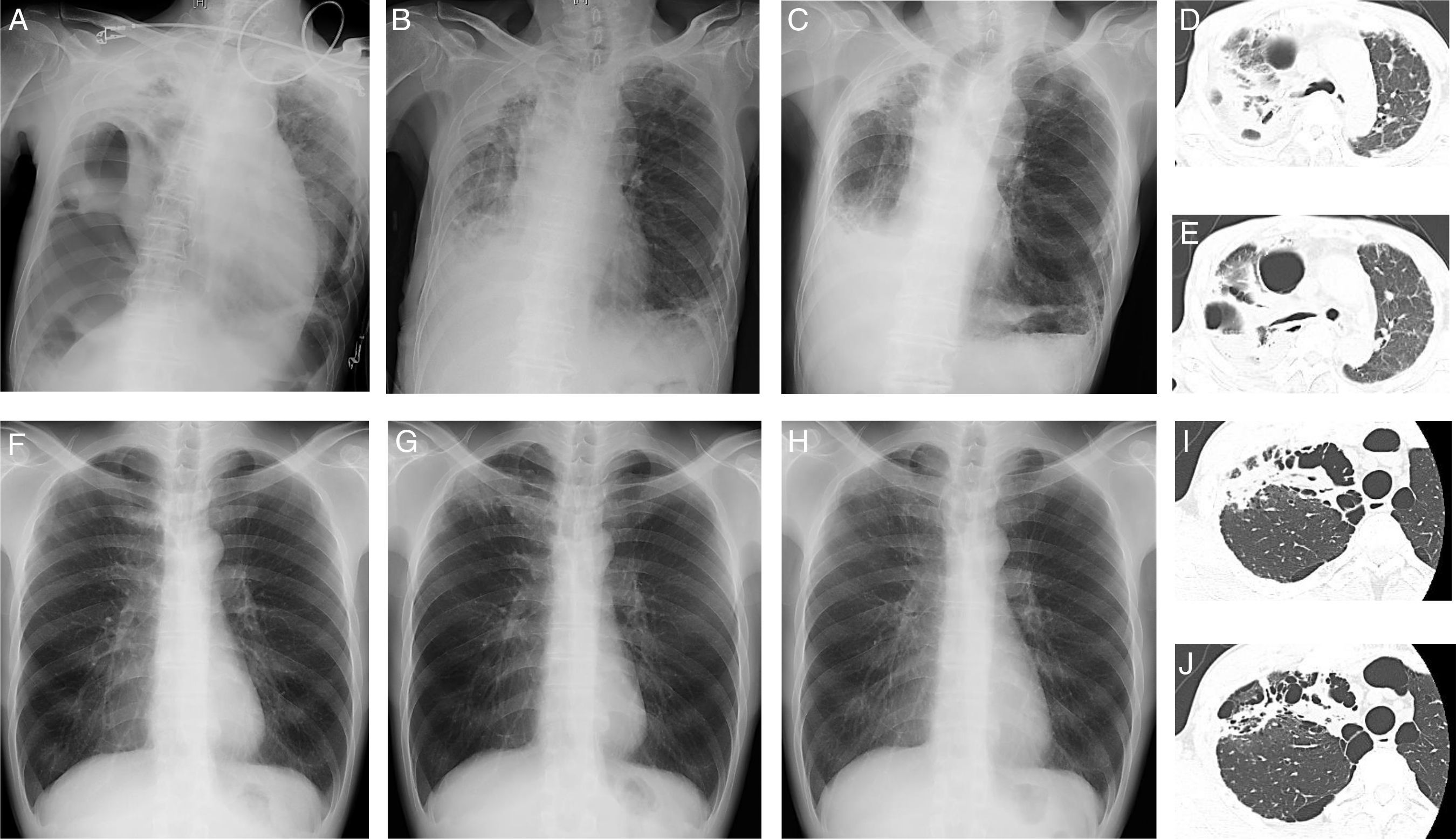
A 86-year-old man (Case 1), an ex-smoker, presented with dyspnea and fever. He had no history of pulmonary tuberculosis. He did not respond to oral levofloxacin, and was referred to Hikone Municipal Hospital for further investigation. His white blood cell count was 16,290/μl and C-reactive protein level was 16.22mg/dL. Chest X-ray and computed tomography (CT) showed pulmonary infiltration with an air bronchogram and bronchiectasis in the right upper lobe and right pneumothorax with pleural effusion (Fig. 1A, D, E). Pleural fluid analysis revealed a neutrophil-predominant (64%) exudate, with 117mg/dL glucose, 770IU/L lactate dehydrogenase, and 57.2U/L adenosine deaminase. Initial blood and pleural fluid cultures were negative for bacterial and mycobacterial organisms. Initial sputum culture was negative for bacterial organisms but positive for M. szulgai, which was identified using a DNA-DNA hybridization method. Empirical antibiotic treatment with 1g cefozopran twice daily was started and thoracic drainage was performed. Because of persistent fever, antibiotic treatment was changed to 150mg ciprofloxacin twice daily at 2 weeks post-admission. The second sputum culture was negative for bacterial organisms but positive for M. szulgai. Furthermore, the second pleural fluid culture was positive for mycobacterial organisms; however, no particular species could be identified. Ciprofloxacin was continued for 1 week, and chest X-ray showed improvement in pleural effusion 1 month post-admission (Fig. 1B). The patient was discharged 47 days after admission. Two months later, repeat chest X-ray showed further improvement in pleural effusion; however, infiltration and bronchiectasis persisted (Fig. 1C).
Chest X-ray images on admission (A), 1 month after admission (B), and 2 months (C) after discharge and chest computed tomography images (D, E) in Case 1. Chest X-ray images at routine medical check-up (F) and 4 (G) and 8 (H) months after routine medical check-up and chest computed tomography images (I, J) in Case 2.
A 44-year-old man (Case 2), a current-smoker, presented with an abnormal chest X-ray at a routine medical check-up. He had no history of pulmonary tuberculosis. Chest X-ray showed apical infiltration of the right lung (Fig. 1F) with significant accumulation on subsequent 18F-fluorodeoxyglucose positron emission tomography/CT. Initial fiber-optic bronchoscopy did not detect any organisms, including bacteria and acid-fast bacilli, or malignant tumors. Despite treatment with 500mg of amoxicillin and 125mg of clavulanic acid 3 times daily for 2 weeks, increased apical infiltration of the right lung was observed on chest X-ray (Fig. 1G) and the patient complained of chest discomfort. Chest CT revealed pulmonary infiltration with suspicious cavitation and bullae in the right upper lobe (Fig. 1I, J). Repeat fiber-optic bronchoscopy 2 months after the initial examination revealed the presence of M. szulgai, which was identified using a DNA–DNA hybridization method. The chest X-ray showed improvement in right lung abnormality 4 months after the second fiber-optic bronchoscopy without antimicrobial treatment (Fig. 1H).
Both cases were diagnosed as pulmonary M. szulgai infection according to American Thoracic Society diagnostic criteria.2 To the best of our knowledge, this is the first case report of pneumothorax associated with pulmonary M. szulgai infection. Pneumothorax is a rare complication of pulmonary infection due to NTM.4 Ueyama et al. reported that patients with pneumothorax due to NTM had advanced disease, a high rate of pneumothorax recurrence, and poor prognosis, regardless of how the pneumothorax was treated.4 Case 1 could only be treated with thoracic drainage. In the future, this patient's disease profile needs to be monitored.
M. szulgaiis rarely isolated from humans, and accounts for just <0.5% of all human isolates of NTM.5 Previous reports have summarized some of the clinical and radiological features of pulmonary M. szulgai infection.5–8 The majority of patients were men over 50 years of age. The most frequent risk factors were chronic alcoholism, smoking, chronic obstructive pulmonary disease, and pulmonary tuberculosis. Chest images commonly showed centrilobular nodules and infiltrate with or without upper lobe cavitation. Our cases had a risk factor, a history of smoking, and infiltrates on chest images; these features are consistent with previous reports.
M. szulgai is characterized by in vitro susceptibility to most standard antituberculosis drugs, such as isoniazid, rifampin, and ethambutol.9 Furthermore, susceptibility of M. szulgai to quinolones, such as ciprofloxacin, levofloxacin, and ofloxacin, and macrolides, including clarithromycin, has been reported.2 Despite the lack of standard recommendations, a 3–4-drug regimen for 12 months after negative sputum cultures is recommended.2 However, when or whether pulmonary M. szulgai infection should be treated remains unknown. In a few cases, similar to Case 2, spontaneous remission has been observed.6 Furthermore, multiple antimicrobial agents can be poorly tolerated and may induce persistent potential adverse effects, such as irreversible vision impairment and kidney function. In addition, the impact of treatment on the patient's overall health status remains unknown.10 Further studies are needed to construct a patient-centered management framework in pulmonary M. szulgai infection.
Here, we describe 2 cases of pulmonary M. szulgai: 1 with pneumothorax and the other with improvement on chest imaging without antimicrobial treatment. This report should highlight the need for a patient-centered management framework in pulmonary M. szulgai infection.