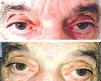
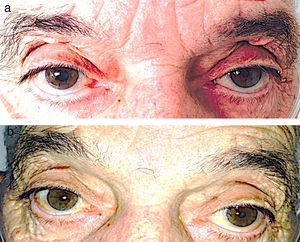
A 75-year-old man with a history of heart valve disease and permanent atrial fibrillation was admitted for severe hypoxemic respiratory failure secondary to bronchial infection by Influenza A H1N1 virus. He was given oseltamivir and nebulized ipratropium bromide (IB) administered via a face mask. One day after admission, anisocoria with a fixed dilated right pupil was seen on physical examination (Fig. 1a). Since the patient was asymptomatic, with no headache, ocular pain or focal neurological signs, we decided to discontinue nebulized IB and wait and see. Twenty-four hours after suspending treatment, the mydriasis had resolved, and the pupils were isocoric and normoreactive (Fig. 1b).
Generalized use of nebulized bronchodilators is steadily increasing, although they have been associated with various complications, including anisocoria when used in combination with IB, as described here, and even more serious adverse effects such as drug-induced angle-closure glaucoma.1 Detecting potentially at-risk patients, evaluating other forms of administration such as mouth pieces, or using protective glasses, are just some important measures worth considering to prevent these complications.2
Please cite this article as: Ferreira-González L, Trigás-Ferrín M, Marcos PJ. «Ojo» a los broncodilatadores en nebulización. Arch Bronconeumol. 2015;51:151.