Chronic hypersensitivity pneumonitis (CHP) is an entity characterized by a destructuring of the lung parenchyma as a result of an inflammation of immunological origin, secondary to the repeated inhalation of an antigen (usually of an organic nature) to which the individual has previously been sensitized.1 In early stages, antigen avoidance can contribute to the resolution of the disease; however, in chronic stages the evolution usually progresses despite antigen avoidance and treatment with systemic corticosteroids, with a 5-year mortality rate of 31%.2,3
Rituximab is a pan-B murine/human chimeric monoclonal antibody which binds specifically to the CD20 membrane antigen and has been used successfully in some interstitial lung diseases (ILDs) associated with collagen diseases.4,5 A case has been described in which rituximab was shown to be effective in the treatment of CHP, obtaining a significant symptomatic improvement with increases of 40% in the CO transfer test (DLCO) and of 15% in the forced vital capacity (FVC).6 In another series of six patients with CHP, functional stability was also observed in three.7
The objective of the present study was to describe the effect of rituximab in a series of five patients with CHP, who presented a progressive loss of pulmonary function despite conventional treatment with systemic corticosteroids and avoidance of contact with the causative antigen.
Five patients diagnosed with CHP who had presented decreases in FVC of more than 10% and/or in DLCO of more than 20% over the previous two years were treated with two doses of 1000mg of rituximab, administered intravenously, separated by an interval of 15 days. This retrospective review describes our experience with these five CHP patients with poor evolution despite conventional treatment, who were treated compassionately with rituximab in accordance with current clinical practice.
The study was authorized by the Ethics Committee of the local hospital. The five patients gave their informed consent to participate.
CHP was diagnosed in accordance with the criteria of Schuyler et al.,8 and patients also fulfilled the currently accepted criteria proposed by Vasakova et al.9 The FVC, DLCO and the 6-minute walking distance (6MWD) were measured at baseline, at 15 days and at 1, 3, 6 and 11 months. A chest high resolution computed tomography (HRCT) was performed at baseline, at 20 days (in order to discard drug toxicity), at 3 months and 11 months after treatment, with assessment of the profusion and/or the appearance of new images.
The median age of patients was 72 years (p25–p75 72–73). All patients were male, and four of the five were ex-smokers (>10packsq/year). They initially had a median FVC of 59% and a median DLCO of 31%, and in the 6MWD they had walked a median of 396m (324–463m). As regards the HRCT, all the patients presented peribronchovascular fibrosis, traction bronchiectasis, mosaic attenuation, air trapping and relative sparing of the bases. Ground glass opacities were minimal or absent in all patients.
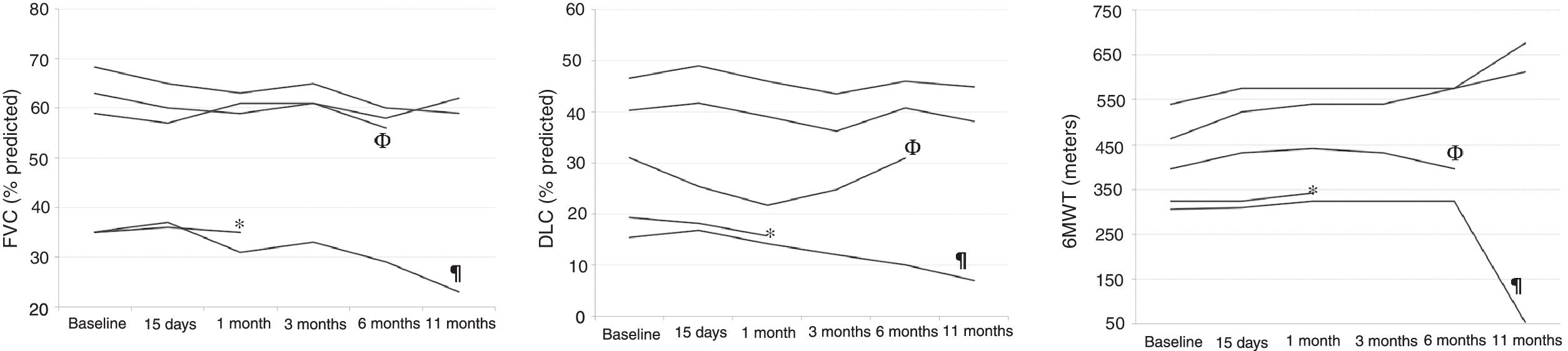
Eleven months after the treatment with rituximab, patients had median decreases of 9.3% (−11.8 to 3.2) in FVC and of 2.2% (−8.4 to −1.7) in DLCO (Fig. 1), and median increases of 72m (−252 to 213m) in 6MWD. There were no significant changes in the HRCT images in any of the patients, at 20 days at 3 months or at 11 months (Fig. 1).
(A) Evolution of the forced vital capacity; (B) evolution of the CO transfer test; (C) evolution of the 6-minute walking distance. Acronyms: FVC, forced vital capacity; DLCO, CO transfer test; 6MWT, 6-minute walking test; *, the patient died at month 3 of follow up; ¶, the patient died at month 11 of follow up; Φ, one patient was unable to perform FVC, DLCO and 6MWT at month 11 due to a high respiratory tract infection.
Two patients died during follow-up; one of them in the third month, and the other, eleven months after rituximab administration. In both cases, death was attributed to progression of the underlying disease. No adverse effects were observed related to the administration of rituximab.
The three patients with an initial FVC greater than 50% and a DLCO greater than 30% did not present significant reductions in these two values during the study period. These three patients raised their 6MWD up to the limit of significance (p<0.06) despite the small sample size. These results seem promising, bearing in mind that both 6MWD and change in 6MWD have been shown to be independent predictors of mortality in other fibrotic ILDs.10
The two patients who presented very low pulmonary function values at the time of treatment (FVC 35% and 34.5%, and DLCO 18% and 15% respectively) were both exitus, at months 3 and 11 of follow-up respectively. In these patients, rituximab did not improve or stop the fall in FVC and DLCO, nor did it improve the distance covered in the 6MWD.
Interestingly, the three patients who presented FVC values of 50% or more and DLCO values of 30% or more at baseline presented an almost significant benefit in the 6MWD, and a stabilization of the lung function values during follow-up after treatment with two doses of rituximab. This suggests that, in early phases of the disease, the addition of rituximab may to some extent help to stabilize the disease. Although it has traditionally been considered that the inflammation in HP is predominantly mediated by T lymphocytes, self-reactive B lymphocytes with the mediation of T lymphocytes may play a decisive role in this inflammatory chain, which would suggest a possible favourable effect of treatment with rituximab in the initial phases of CHP.11
No infectious complications (opportunistic infections, fungal infections or isolation of mycobacteria) were identified related to the administration of rituximab. These data coincide with other studies of patients with ILDs treated with rituximab, and confirm the relative safety of the use of the drug in this subgroup of patients, although close monitoring is mandatory.
The small number of patients and the absence of a control group mean that no definitive conclusions can be obtained. However, the favourable evolution presented by other sporadic cases6,7 and by the patients described here suggests that rituximab may be of some utility in a subgroup of patients with CHP who do not respond to antigen avoidance and treatment with systemic corticosteroids. Randomized controlled clinical trials should be carried out in order to confirm these encouraging initial findings.
FundingBeca Fundacio Catalana de Pneumologia (FUCAP) and Fundación Cellex.
Conflicts of interestThe pharmaceutical industry did not participate in the conception, nor in the financing of the study. IO has received consulting fees, talk fees or research grants from Astrazeneca, Boehringer Ingelheim, GlaxoSmithKline, Mundipharma, Novartis and TEVA. AV has received consulting fees, talk fees or research grants from Boehringer Ingelheim, GlaxoSmithKline and Roche. XM has received consulting fees, talk fees or research grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Faes, GlaxoSmithKline, Menarini, Mundifarma, Novartis and Teva.