Allergic bronchopulmonary aspergillosis (ABPA) is a complex hypersensitivity reaction in response to colonization of the airways with Aspergillus fumigatus.1 It occurs primarily in patients with asthma or cystic fibrosis (CF).2,3 We report a case of this disease without asthma or CF, but with an elevated carcinoembryonic antigen (CEA) instead.
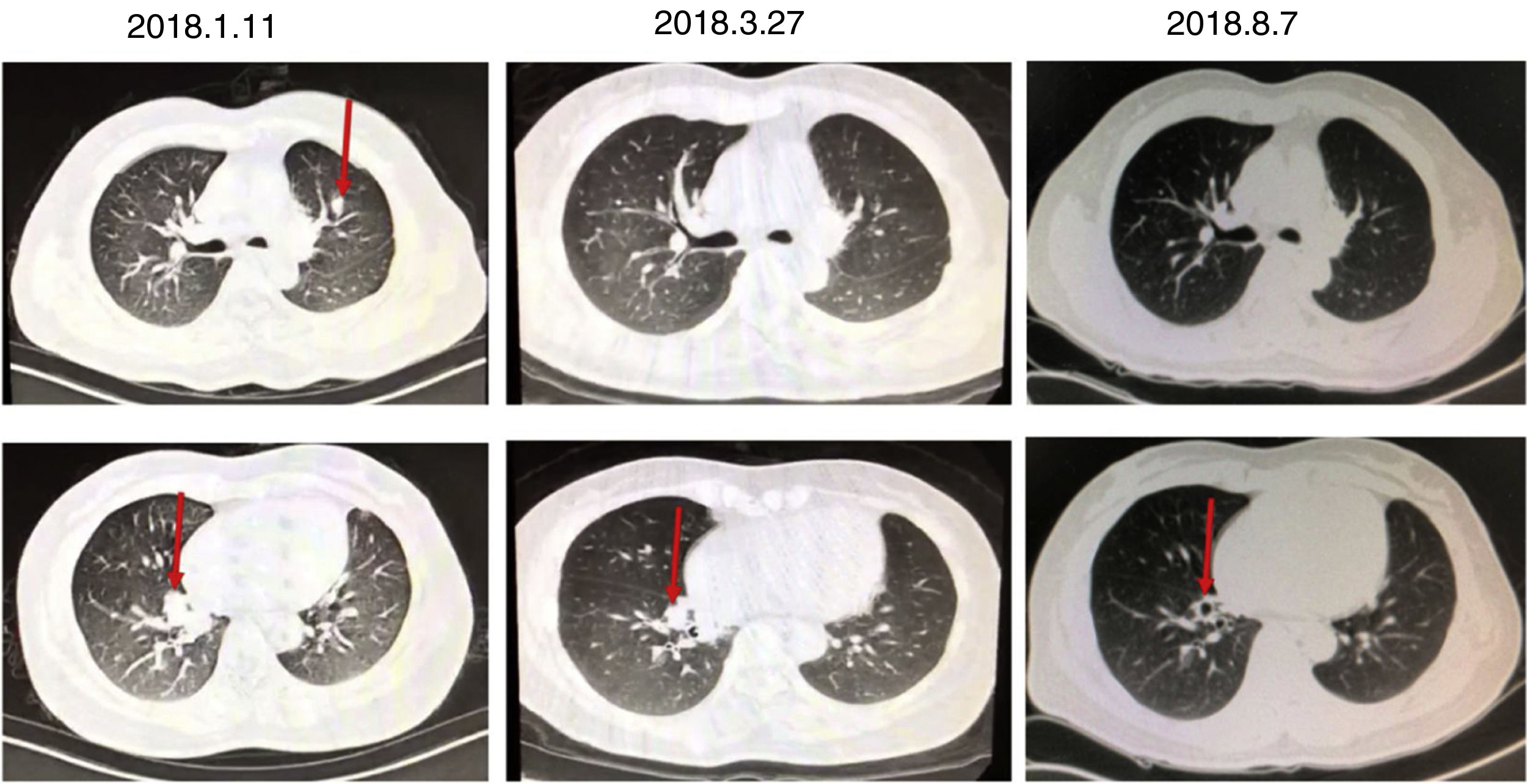
A 46-year-old woman, non-smoker, with no history of asthma or recurrent lung disease was admitted to the hospital for paroxysmal cough for two months. She denied expectoration, shortness of breath, chest pain, hemoptysis, fevers or chills. During the course of her illness, the patient lost 2.5kg of weight with poor appetite. She has a surgical history of lingular segment of the left upper pulmonary lobe resection in 2015, and the histopathological report showed bronchiectasis, acute infections and abscesses. Laboratory data revealed a markedly elevated blood eosinophil count and percentage (1.2×109/L, 15.1%) and total serum level of immunoglobulin E (1412kU/L). Chest CT revealed shadow in the medial basal segment of the right lower lobe and the upper left lung, right lung bronchiectasis and infection on 11th January 2018 (Fig. 1). In considering of her surgical history and chest CT, we tested several tumor markers to rule out primary and metastatic tumors. The serum CEA (24.2ng/mL) was remarkable elevated, while serum CA19-9 level was normal. A subsequent PET scan showed no focal increase in fluorodeoxyglucose activity. Bronchoscopy showed inflamed bronchial mucosa and cytological examination of bronchoalveolar lavage fluid showed no cancer cells nor heterotypic cells. Pulmonary function test results showed a vital capacity of 2.87L (75.3% of predicted), FVC of 4.14L (80% of predicted), FEV1 of 2.15L (84.5% of predicted), FEV1/FVC ratio of 74.86%. According to the patient's pulmonary function at that time, when the cumulative dose of acetylmethcholine reached 2.5mg (12.8 micromole), FEV1 decreased by 11.0% in the patient. Bronchial histamine provocation test and bronchodilator test were negative. She was limited response to levofloxacin treatment. Subsequently, the results of A. fumigatus-specific IgE 8.26kUA/L, A. fumigatus-specific IgG>500AU/mL. One month oral corticosteroid treatment was helpful for improving patient's symptom and radiological abnormality (Fig. 1). Total IgE concentration, which is a key indicator for assessing the treatment response and relapse, was rapidly declined after 3 months treatment associated with symptomatic improvement. The concentration of serum CEA decreased to normal range 6 months later. No relapse has been observed throughout the one-year follow-up (Table 1).
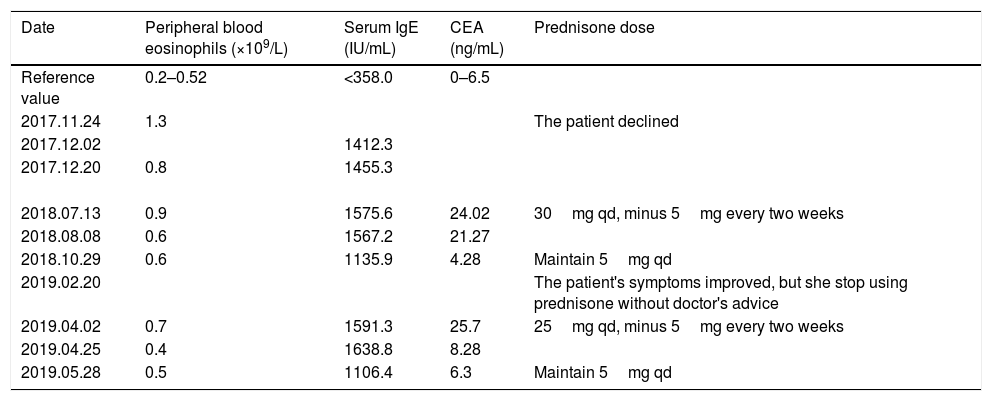
The course of treatment and dose of prednisone, as well as the data of CEA, IgE, and peripheral blood eosinophils before and after the treatment.
| Date | Peripheral blood eosinophils (×109/L) | Serum IgE (IU/mL) | CEA (ng/mL) | Prednisone dose |
|---|---|---|---|---|
| Reference value | 0.2–0.52 | <358.0 | 0–6.5 | |
| 2017.11.24 | 1.3 | The patient declined | ||
| 2017.12.02 | 1412.3 | |||
| 2017.12.20 | 0.8 | 1455.3 | ||
| 2018.07.13 | 0.9 | 1575.6 | 24.02 | 30mg qd, minus 5mg every two weeks |
| 2018.08.08 | 0.6 | 1567.2 | 21.27 | |
| 2018.10.29 | 0.6 | 1135.9 | 4.28 | Maintain 5mg qd |
| 2019.02.20 | The patient's symptoms improved, but she stop using prednisone without doctor's advice | |||
| 2019.04.02 | 0.7 | 1591.3 | 25.7 | 25mg qd, minus 5mg every two weeks |
| 2019.04.25 | 0.4 | 1638.8 | 8.28 | |
| 2019.05.28 | 0.5 | 1106.4 | 6.3 | Maintain 5mg qd |
CEA, is known to be one of the most extensively used clinical marker for colorectal carcinoma and lung cancer.4 It can be produced in the epithelium of the stomach, intestines, bile ducts, and respiratory tract ranging from the trachea to the alveoli and plays a role in the progress of cell adhesion and participate in the innate immune defense.5 Noguchi et al. measured serum CEA levels in patients with ABPA and found serum CEA levels are elevated in some patients with ABPA, which might be associated with consolidation in the lung.6 Elevated serum CEA levels decrease as the consolidation decreases after treatment. The elevation of serum CEA might be attributed to the presence of local inflammation in the lung. We found several cases reported asthmatic patients with high level of CEA in serum and bronchoalveolar lavage fluid might be related to the mucoid impaction.7,8 In our case, we found the level of CEA was not directly related to the mucoid impaction in our follow-up. Tang et al. have discovered that the increase level of CEA was related to the increase number of eosinophil granulocytes, and corticosteroid therapy was effective in improving clinical symptoms and the CEA values decreased in association with the improvement of those manifestations, suggesting a pathophysiological link between the disease activity of hypereosinophilia and the changes in CEA level.9,10 In our case, we found that the level of CEA decreased along with the decrease of eosinophil granulocytes. The underlying mechanism remains unclear. Besides, there is also a report showing that a 21-year-old man with bronchial asthma who suffered from productive cough had an elevated serum CA19-9 level.11 In that case, immunohistochemical studies showed no expression of CA19-9 in bronchial biopsy specimens, but the CA19-9 level recovered to normal range after steroid therapy. This indicates that the high serum concentration of CA19-9 was probably due to hypersecretion of mucus glycoprotein, secreted from hypertrophic glands or/and epithelial cells in the bronchioles. These finding suggests that CEA is correlated with the inflammatory activity of ABPA while the role of CEA elevation remains unclear. Larger numbers of cases should be collected and evaluated.