Eosinophilic granulomatosis with polyangiitis (EGPA) is a systemic vasculitis of the small and medium-sized vessels, the pathogenesis of which is still unknown. Occurring at a prevalence of 10–13 cases per million, it is characterized by a variety of clinical and laboratory abnormalities, and 40% of cases are associated with MPO pANCA antibodies.1–4
Eosinophils play a key role in the pathophysiology of this disease, by causing local tissue injury and persistent Th2 inflammation due to the release of granular proteins and cytokines, including IL-5. IL-5 selectively stimulates the eosinophil lineage in the bone marrow, and plays a central role in the differentiation, survival and proliferation of peripheral eosinophils, fostering their adhesion to the vascular endothelium.4 The anti-IL 5 antibody mepolizumab is a therapeutic alternative that inhibits IL-5 binding with its receptor on the surface of eosinophils, thus decreasing the production and survival of these cells.5–8 Mepolizumab has proven to be an effective treatment in patients with EGPA at an intravenous dose of 750 mg/month7–9 and subcutaneous doses of 300 mg/month.5 We report the cases of 3 patients diagnosed with EGPA who responded to mepolizumab at a subcutaneous dose of 100 mg/month.
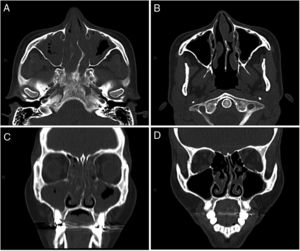
The first was a 34-year-old woman with a history of pansinusitis and difficult-to-control asthma. Since 2006 (when she was 21 years old), she has had frequent episodes of bronchospasm with consultations in the emergency room and two admissions to intensive care. In 2011, she was hospitalized for acute abdominal pain, and clinical laboratory tests showed peripheral eosinophilia (518 cells/mm3, raised IgE, and positive MPO pANCA. Endoscopic biopsy was performed which revealed eosinophilic gastroduodenitis. EGPA was diagnosed, and treatment began with meprednisone and azathioprine, which was discontinued due to osteonecrosis of both knees and severe leukopenia. She was subsequently treated sequentially with cyclophosphamide, omalizumab, and rituximab. Progress was poor, with poorly controlled asthma, persistent pansinusitis, recurrent cutaneous vasculitis, weight loss, and raised eosinophils 1,224 cells/mm3). In August 2016 she started mepolizumab 100 mg/month, with frank clinical and radiological improvement (Fig. 1), and a reduction of eosinophils to 46 cells/mm3, maintained to date.
Computed tomography of the sinuses, axial (A and B) and coronal (C and D) slices. A and C: before treatment with mepolizumab: left septal deviation. Marked mucosal thickening of both maxillary sinuses, ethmoidal air cells, and both nostrils. Exudate in both maxillary sinuses. B and D: after 4 months of treatment with mepolizumab: minimum mucosal thickening in the maxillary sinuses. Mucosal thickening and polypoid formations of both nostrils.
Our second patient was a 59-year-old woman with a history of chronic rhinosinusitis, hereditary angioedema, and severe asthma. In 2011 51 years old she was seen for fever, radiological pulmonary infiltrates, and peripheral eosinophilia 4,128 cells/mm3), with negative ANCA, which responded well to meprednisone. In 2013 she was hospitalized with fever, pulmonary infiltrates, myocarditis, and pericardial effusion. EGPA was suspected, and the patient was prescribed meprednisone and azathioprine. She continued to present poorly controlled asthma, peripheral eosinophilia, moderate obstruction on spirometry, and slightly reduced diffusion capacity. In May 2018, she started mepolizumab 100 mg/month, with sustained clinical and functional improvement.
Our third patient was a 49-year-old woman with a history of chronic rhinosinusitis and bronchial asthma. In 2010 40 years old, she was seen for fever and dyspnea, and diffuse alveolar-interstitial pulmonary infiltrates, peripheral eosinophilia 1,802 cells/mm3), raised IgE, and positive MPO pANCA were detected. The diagnosis was EGPA, and she received meprednisone, but relapsed when systemic steroids were discontinued. She was treated sequentially with steroids plus azathioprine for 2 years, omalizumab for 1 year, and rituximab for 1 year. The patient remained symptomatic, so treatment began with mepolizumab 100 mg/month in September 2018. She showed prompt and sustained clinical and functional respiratory improvement.
EGPA is a systemic vasculitis that affects small and medium-sized vessels, and is associated with generally difficult-to-control asthma. It requires induction and maintenance treatment with steroids and immunomodulators, relapses are common, and adverse effects are potentially serious. The anti-IL-5 antibody mepolizumab has recently been evaluated as a new therapeutic alternative with promising results. Wechsler et al.5 reported a trial in which mepolizumab was administered subcutaneously at a dose of 300 mg every 4 weeks and was associated with a lower relapse rate and longer accumulated time in disease remission than the placebo group, allowing glucocorticoid doses to be reduced.
The only randomized, placebo-controlled trial published5 used mepolizumab doses of 300 mg/month in patients with EGPA, without assessing the impact of lower doses, and without citing references to justify this dose. Pouliquen et al.10 evaluated the response of eosinophils in blood to escalating doses of mepolizumab, showing a maximum 90% inhibition in peripheral eosinophils at 12 weeks with the subcutaneous dose of 99 mg mepolizumab.
Our 3 patients were treated with a subcutaneous dose of 100 mg/month, and achieved reductions in their peripheral eosinophil count and improved pulmonary and extrapulmonary manifestations, allowing them to discontinue systemic steroids. During the follow-up period, these patients maintained this improvement, developed no relapses, and required no increase in dose.
The number of patients in our publication is limited, but it opens up the possibility of starting treatment with mepolizumab at lower doses in patients with EGPA.
Please cite this article as: Moyano Viviana A, et al. Granulomatosis eosinofílica con poliangeítis tratada con mepolizumab en dosis de 100 mg/mes. Arch Bronconeumol. 2020;56:253–254.