The diagnosis of idiopathic pulmonary fibrosis (IPF) is a complex process that requires the multidisciplinary integration of clinical, radiological, and histological variables. Due to its diagnostic yield, surgical lung biopsy has been the recommended procedure for obtaining samples of lung parenchyma, when required. However, given the morbidity and mortality of this technique, alternative techniques which carry a lower risk have been explored. The most important of these is transbronchial cryobiopsy—transbronchial biopsy with a cryoprobe—which is useful for obtaining lung tissue with less comorbidity. Yield may be lower than surgical biopsy, but it is higher than with transbronchial biopsy with standard forceps. This option has been discussed in the recent clinical guidelines for the diagnosis of IPF, but the authors do not go so far as recommend it. The aim of this article, the result of a multidisciplinary discussion forum, is to review current evidence and make proposals for the use of transbronchial cryobiopsy in the diagnosis of IPF.
El diagnóstico de la fibrosis pulmonar idiopática (FPI) es un proceso complejo que precisa la integración multidisciplicinar de variables clínicas, radiológicas e histológicas. Cuando es preciso obtener muestras de parénquima pulmonar, la biopsia pulmonar quirúrgica ha sido el procedimiento recomendado por su rendimiento diagnóstico. Pero dada la morbi-mortalidad de esta técnica, se han explorado alternativas con menores riesgos.. La más importante es la biopsia transbronquial con criosonda (criobiopsia transbronquial), que permite obtener tejido pulmonar con menor comorbilidad, con un rendimiento inferior a la biopsia quirúrgica pero superior a la biopsia transbronquial con pinza convencional. Por ello, en las recientes guías clínicas para el diagnóstico de la FPI se ha valorado esta opción, sin llegar a obtener una recomendación. En este artículo, resultado de un foro de discusión multidisciplinar, se pretende revisar la evidencia actual y hacer propuestas sobre el uso de la criobiopsia transbronquial para el diagnóstico de la FPI.
The diagnosis of idiopathic interstitial pneumonias (IIP) is based on the multidisciplinary analysis of clinical, radiological and histologic data.1 The conventional procedure of choice for obtaining samples of lung tissue is surgical lung biopsy (SLB) because specimens obtained using standard transbronchial biopsy (TBB) are unsuitable for the diagnosis of these pathologies.2 Transbronchial cryobiopsy (TBCB) is a method for obtaining lung tissue using endoscopic cryoprobes.3 The use of this technique in the study of IIP has expanded in recent years as an alternative to SLB.4 It has also been proposed in patients with idiopathic pulmonary fibrosis (IPF), but the recently published ATS/ERS/JRS/ALAT clinical practice guidelines conclude that scientific evidence is insufficient to recommend its use.5
In this article we aim to initiate a broader, multidisciplinary discussion on the value of TBCB in patients with suspected IPF and to propose a possible diagnostic schema incorporating this technique.
Diagnosis of idiopathic pulmonary fibrosis. What do the current guidelines propose?The diagnostic process in idiopathic pulmonary fibrosisTwo major documents were published on the diagnosis of IPF in 2018: the updated ATS/ERS/ALAT/JRS diagnostic guidelines and the Fleischner Society White Paper.5,6 According to current recommendations, the diagnosis of IPF is based on the findings of a multidisciplinary assessment, the appropriate clinical context (more prevalent in patients older than 60 years, men, and former smokers), and a pattern of usual interstitial pneumonia (UIP), either on high-resolution computed tomography (HRCT) or analysis of the histologic material obtained by needle biopsy of the lung.5,6 The process, therefore, aims to:
- •
Rule out other possible causes of the origin of the lung disease through a comprehensive clinical history and complementary tests, and exclude systemic autoimmune disease, occupational or domestic exposures, and drugs with potential pneumotoxicity7;
- •
Confirm the presence of a pattern of UIP according to accepted radiological and histologic criteria2.
An important feature of the 2002 guidelines that has been given particular emphasis in the most recent versions is that multidisciplinary assessment is the “gold standard” in the diagnostic process. This reflects a fundamental change from the previously established criteria in which the histologic pattern was given greater weight than all other factors.8 Furthermore, priority is given to less invasive diagnostic processes, with biopsy being reserved for cases that are inconclusive after the initial multidisciplinary assessment.
Role of high-resolution computed tomography in the diagnosis of idiopathic pulmonary fibrosisLeading clinical guidelines regard HRCT as an essential component in the diagnosis of IPF, as it can identify UIP from: (a) evidence of fibrotic changes in both lungs, predominantly in the lung bases and subpleural regions, despite a frequent patchy pattern; (b) reticular opacities, often associated with traction bronchiectasis; (c) honeycombing manifested as clumped cystic spaces (diameters of between 3 and 10mm); and (d) the absence of other findings listed as inconsistent for UIP.5
The first step in the diagnosis of IPF, then, is the use of an appropriate technique that allows us to obtain images of high diagnostic quality. The essential differences between conventional CT and HRCT reside in the slice thickness used in the acquisition and reconstruction of the images.9,10 In HRCT, images are obtained from 1mm slices, and (as in conventional CT) with the patient in a supine position at deep inspiration. A high frequency algorithm and a field of vision limited to the pulmonary parenchyma are used for image reconstruction.11 Intravenous contrast is not administered during the HRCT. The images obtained in expiratory series are very useful for demonstrating air trapping.12,13
Due to inter-observer variability and discrepancies, and advances in disease characterization, imaging techniques, and the collection of histologic samples using cryobiopsy, the Fleischner Society has published a number of new radiological criteria for diagnosing IPF and facilitating access to treatment.6 In this review, 4 new categories differentiating UIP patterns in HRCT have been proposed: typical, probable, and indeterminate UIP patterns, and alternative diagnoses to IPF (Table 1). The “possible” pattern has been changed with respect to the previous classification to “probable” UIP, and “inconsistent” has been changed to “indeterminate”.
UIP diagnostic categories based on HRCT.
| Typical UIP pattern | Probable UIP pattern | Indeterminate UIP pattern | Findings consistent with a diagnosis other than IPF | |
|---|---|---|---|---|
| Distribution | Predominantly basal (occasionally diffuse) and subpleural; often heterogeneous | Predominantly basal and subpleural; often heterogeneous | Variable or diffuse | Fibrosis predominantly in upper lobes or upper or middle lung; predominantly peribronchovascular with normal subpleural space. |
| Findings | Honeycombing; reticular pattern with peripheral distribution of traction bronchiectasis or bronchiolectasis; no findings suggestive of an alternative diagnosis | Reticular pattern with peripheral distribution of traction bronchiectasis or bronchiolectasis; no honeycombing; no findings suggestive of an alternative diagnosis | Evidence of fibrosis with some discrete features suggestive of a non-NIU pattern | Any of the following: predominant consolidation, ground glass opacity (without acute exacerbation), extensive mosaic attenuation pattern, attenuation with significant lobular air trapping in expiration, diffuse nodules or cysts |
HRCT: high-resolution computed tomography; IPF: idiopathic pulmonary fibrosis; UIP: usual interstitial pneumonia.
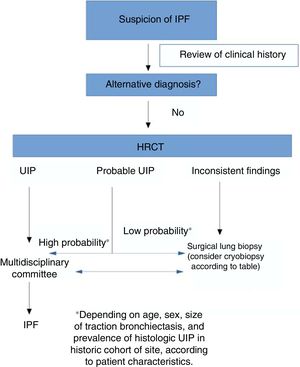
According to our review, an accurate diagnosis of IPF can be made when HRCT images show a pattern of typical or probable UIP.14 If the clinical context is indeterminate for IPF or the HRCT pattern is not indicative of typical or probable UIP, completing the diagnostic process with lung biopsy should be considered.
The updated ATS/ERS/JRS/ALAT guidelines also propose 4 radiological patterns in the diagnosis of IPF that are similar but not identical to those proposed by the Fleischner Society.5,6 The basic findings of UIP and probable UIP patterns are similar, but the indeterminate pattern differs by including the presence of predominantly basal subpleural reticulation (also known as “early UIP”), along with the true indeterminate pattern (a pattern of fibrosing interstitial lung disease with findings that really do not guide the clinician towards any etiology).
One of the main differences between the ATS/ERS guidelines and the Fleischner Society document lies in the recommendation for performing or not performing lung biopsy in patients with a pattern of possible UIP in a clinical context consistent with IPF: the ATS/ESR guidelines make a conditional recommendation in favor of this procedure.14 In short, then, for the authors of the Fleischner guidelines, the risk of comorbidities associated with SLB is greater than the benefit of finding an alternative diagnosis in patients with a high clinical suspicion of IPF and HRCT showing a pattern of probable UIP. This is based on various studies published in recent years that have analyzed the correlation between the probable UIP pattern and the presence of UIP in lung biopsy.15,16 As the study of Brownell et al. clearly shows, in cohorts with a high prevalence of histologic UIP, the pattern of possible UIP on HRCT is sufficient to predict this diagnosis and hence avoid SLB.17 However, in other situations, the positive predictive value is lower, so we need other clinical variables (such as sex, age, or presence of traction bronchiectasis) to identify groups of subjects with a high positive predictive value for histologic UIP. As such, the pretest probability is very important for evaluating the radiological pattern of possible UIP. TBCB could be an excellent alternative in this situation, since it has less comorbidity than SLB, and while the diagnostic yield is lower than that of SLB, it may be sufficient to substantiate the diagnosis of IPF in cases with a pattern of probable UIP.
Histologic diagnostic criteria for usual interstitial pneumoniaThe characteristics of pulmonary parenchyma in a biopsy with a UIP pattern were established in various consensus documents, and have been slightly modified in subsequent updates published in 2011, 2013, and most recently, 2018.1,5,18 The latest 2018 guidelines describe the following histologic characteristics:
- •
Evidence of interstitial fibrosis associated with marked distortion of the lung architecture with a characteristic distribution of predominantly peripheral involvement in the secondary lobule, i.e. paraseptal or subpleural.
- •
Presence of fibroblast foci in areas of interface of fibrosis with normal parenchyma.
- •
Presence of heterogeneous lesions that result in a combination of fibrotic areas of advanced involvement and areas with normal alveolar septa.
- •
Absence of features inconsistent with a diagnosis of UIP, suggesting an alternative diagnosis. Despite showing all the histologic characteristics of UIP, the presence of granulomas or other specific features that correspond to another entity rule out the diagnosis.
These characteristics can be difficult to comprehend, so the above-mentioned 2018 guidelines provide 4 options on the probability of a UIP diagnosis. These are listed in Table 2 following the same format as Table 1.
Classification of lung biopsies.
| UIP pattern | Probable UIP pattern | Indeterminate UIP pattern | Alternative diagnosis |
|---|---|---|---|
| 1. Architectural distortion with dense fibrosis (honeycombing) | 1. Incomplete characteristics of the first column plus absence of findings suggestive of an alternative diagnosis | 1. Fibrosis with or without architectural distortion, with characteristics of other patterns or suggestive of other causes | 1. Findings of other histologic patterns of IIP (e.g., no fibroblast foci or loose fibrosis) in all biopsies |
| 2. Prevalence of fibrosis in subpleural or paraseptal area | or | 2. Any characteristic of the first column but with findings suggestive of another diagnosis | 2. Findings suggestive of other disease (e.g., sarcoidosis, HP, LCH, LAM) |
| Presence of honeycombing only | |||
| 3. Heterogeneous distribution of fibrosis | |||
| 4. Fibroblast foci |
LAM: lymphangioleiomyomatosis; LCH: Langerhan’s cell histiocytosis; HP: hypersensitivity pneumonitis; IIP: idiopathic interstitial lung diseases; UIP: interstitial usual pneumonia.
The concept of TBCB arose from the introduction of modified flexible cryotherapy probes and the need to improve the diagnostic yield of TBBs, whose role in the diagnosis of certain diffuse interstitial lung diseases (ILD) was often questioned.1,18 With regard to standardization of the procedure, a recently published expert consensus analyzes the scientific evidence on the diagnostic yield and safety of TBCB in the most relevant published studies.19 In this review, the diagnostic yield of TBCB was within the range of 50.6 %–100 % compared to the 25 %–65 % yield obtained with conventional forceps; pneumothorax occurred in 1.4 %–26 % of cases and bleeding in 1 %–12 %. The authors recommend performing the technique with orotracheal intubation under deep sedation or general anesthesia, using an occlusion balloon to control bleeding under fluoroscopic guidance. They also recommend obtaining 3–5 biopsies in different lung segments, at least 1cm from the pleura.
The first study to demonstrate the viability of TBCB in the diagnosis of ILD was published in 2009, and showed that larger and better quality lung parenchyma samples were obtained compared with TBB. In this initial study, the mean size of the samples was 15.11mm2 compared with 5.82 mm2 for samples obtained with the conventional technique.3 The first Spanish study on the use of TBCB appeared in 2010.20 The diagnostic yield of TBCB vs TBB in ILD was subsequently evaluated in a randomized, prospective study.21 A histopathologic diagnosis was reached in more cases in the TBCB group (74.4 %) than in the TBB group (34.1 %, p<0.001), and the final multidisciplinary diagnosis was higher (51.3 % versus 29.1 %, p=0.038). With regard to IPF, Tomassetti et al. retrospectively assessed the impact of TBCB versus SLB in the multidisciplinary diagnosis of IPF.22 The study showed that the increase in diagnostic precision achieved with the addition of TBCB was similar to that achieved with surgical biopsy in the diagnosis of IPF (SLB from 29 % to 63 %, p=0.0003 and TBCB from 30 % to 65 %, p=0.0016).
Iftikhar et al. conducted a review of 16 studies with TBCB versus 14 studies with SBL that included more than 2200 patients.23 In this meta-analysis, a lower diagnostic yield was observed in the TBCB group (83 %) than in the VATS-SLB group (92 %). In a prospective study, Romagnoli et al. analyzed the diagnostic concordance of TBCB compared to SLB.24 In this study, 21 patients underwent both techniques on the same day, in the same operating room, during the same anesthesia. The results of this study show that the same histologic diagnosis was obtained with both procedures in only 8 out of 21 patients (38 %): SLB yielded larger specimens and was superior in establishing useful histologic patterns in multidisciplinary diagnostics. However, it should be noted that the primary outcome (histologic diagnostic concordance) was obtained from a pathology evaluation that was “blind” to the clinical and radiological information, which is not standard clinical practice. Another significant point is that in this study only 21 of the 61 selected patients were included. That is to say, 40 patients could not be included in the study, either because they refused an SLB or because they were ruled out due to comorbidities.
Another argument in favor of TBCB is the reduced cost compared to SLB by video-assisted thoracoscopy (VATS). Cost analyses found a potential cost savings for TBCB compared to VATS-SLB of between £210-£647 per patient/year25 or between €953.09/patient when the VATS-SLB is performed on an outpatient basis and a maximum of €1,925.29/patient when the SLB involves a 48-h admission.26
With regard to the contraindications for the test, according to the expert consensus, there is no age limit for TBCB, provided it is not ruled out due to comorbidities and lack of fitness for anesthesia.19 It has also been reported that patients with worse lung function have more complications. FVC<50 % predicted or DLCO<35 % predicted, if present, are recommended as relative contraindications. The presence of pulmonary arterial hypertension (PAH) may increase the risk of bleeding, and in some series PAH greater than 40−45mmHg has been regarded as a relative contraindication.3,21
Therefore, although SLB is the method of first choice, TBCB is an alternative when a patient refuses SLB or when SLB is inadvisable.
Surgical lung biopsySLB is still the “gold standard” for the histologic diagnosis of diffuse ILD. Currently, the approach of choice is VATS, using 1, 2, or 3 ports of entry for introducing the camera and instruments.27 The implementation of minimally invasive surgery has helped simplify the technique.28 With the help of HRCT, the biopsy site is selected by multidisciplinary decision, thus improving the cost-effectiveness of the technique. The resection is performed using mechanical endosutures, a technique that is increasingly safe and allows the parenchyma to be simultaneously sectioned and sealed. Samples are sent for histopathological study and microbiological culture. A sample should generally measure about 3×2×1cm3, although some authors recommend a diameter of more than 2cm. SLB is usually performed under general anesthesia with selective intubation, although it is true that there is a growing trend towards performing the procedure with orotracheal intubation or even under sedation with a laryngeal mask.29,30 Endosutures are so safe that postoperative air leak has been reduced to a minimum, so systematic pleural drainage placement is not required.31,32 In outpatient surgery programs, patients are admitted 1−2hours before surgery and discharged about 4−6hours after the procedure. Publications on outpatient surgery programs have reported admission rates of 3 % (mostly due to air leakage) and readmission rates of 3 %, also for pneumothorax.33 A recent prospective, multicenter study involving 224 patients and 13 thoracic surgery departments assessed the feasibility of the outpatient approach in VATS-SLB in the diagnosis of diffuse ILD. The results showed that the anatomical site and the number of biopsies do not influence the diagnosis of ILD: a specific diagnosis was obtained in 87 % of cases (of which 26.1 % were IPF), and morbidity rates after discharge were comparable among patients who were admitted (9/154: 5.8 %) and those who were treated as outpatients (3/70: 4.3 %).34
Open questionsCan a diagnosis of usual interstitial pneumonia in a biopsy be diagnosed by cryobiopsy?TBCB is especially useful in diseases with peribronchovascular and lymphangitic distribution and in diseases with very specific or characteristic features, but it is of little (or less) use in diseases where a pattern must be identified, such as IPF. Despite this, TBCB is gaining utility.
The histologic criteria for UIP were established with SLB, but these features can also be identified in TBCB. The presence of pleura or septum in the sample is necessary to determine the UIP pattern, since the distribution of fibrosis is 1 of the 4 requirements. Therefore, cryobiopsies that do not contain these structures will only be able to establish a “probable UIP pattern”. However, a patchy distribution and higher proliferation of fibroblast foci are easily identifiable in TBCB. Another very characteristic morphological element of the UIP pattern is honeycombing. The presence of honeycombing has been much debated in the literature, as it cannot always be detected. A recent study concluded that areas of histologic honeycombing can be detected in TBCB when more than 2 fragments containing pleura are obtained. The size of the fragments may make it difficult to differentiate between honeycombing and bronchiolar metaplasia.
TBCB, therefore, might not satisfy all the criteria needed to definitively establish a pattern of UIP, but it can still be very useful for identifying histologic characteristics of probable or possible UIP pattern, and for detecting other histologic features that will exclude UIP. For this reason, minimum quality criteria must be established in terms of the number and size of samples, their distribution, and histologic findings. At the microscopic level, some authors suggest that a minimum sample size of 5mm of well expanded parenchyma is needed identify a pattern, and although smaller fragments may contain all the elements, this would be the optimal size.35
SafetyIn terms of safety, the complications most frequently described in association with TBCB are severe bleeding and pneumothorax.4 With regard to bleeding after the procedure, there is no consensus on the definition and quantification of bleeding, so this outcome is very heterogeneous in the literature. In general, studies comparing this technique with TBB have shown a higher percentage of moderate bleeding in the TBCB group than in the SLB group. Johannson et al. performed a meta-analysis in which they found an overall frequency of moderate/severe bleeding of 39 %, with a range of 0%–78%, demonstrating wide heterogeneity.36 In their systematic review, Ganganah et al. compared TBCB with TBB and identified 3 studies that compared directly in terms of frequency of bleeding; bleeding was significantly greater in TBCB in only 1 of the 3.25 Rates of moderate bleeding of 20.99 % (5.6 %–42.8 %) associated with TBCB have also been reported, including 3 deaths (0.5 % of the group).37 In this respect, the recommendation on standardization of the TBCB procedure includes the use of an occlusion balloon to control possible bleeding.19
Sharp et al. conclude that pneumothorax is the most common adverse effect in TBCB, occurring in 20 % of cases (15 % required drainage).37 The total combined probability of presenting pneumothorax was 0.06 (95 % CI: 0.02–0.11), while the probability of pneumothorax with chest tube placement was 0.03 (95 % CI: 0.01–0.08). In studies that show a higher rate of pneumothorax, this finding may be related to the characteristics of the study patients (patients with interstitial lung disease and fibrotic pattern) and the collection of a greater number of subpleural biopsies (<1cm).22,38
With regard to SLB, the incidence of complications in the published series varies widely, the most frequent being postoperative pain, and the most feared being exacerbation of the underlying disease. Both pain and exacerbations have diminished with the implementation of minimally invasive techniques.39,40 In 2007, Kreider et al. reported that the implementation of VATS programs did not reduce morbidity or mortality associated with SLB.41 Careful selection of patients and the time when biopsy is indicated appear to be important factors in reducing complications. Hutchinson et al. published a retrospective analysis which found that mortality associated with non-elective surgery was as high as 16 %, but fell to 1.7 % in elective surgery.42
In meta-analysis reviewing both techniques, the incidence of grade 2 (moderate to severe) bleeding in the TBCB group was 4.9 % (2.2 %–10.7 %).23 In the studies that evaluated VATS-SLB, only 5 reported persistent air leak, at an incidence of 2 % (0.9 %–4 %). In these studies, mortality at 30–60 days post-procedure was 0.7 % (0.4 %–1.2 %) after TBCB and 1.8 % (1.0 %–3 %) after VATS-SLB. Other safety indicators in the comparison of TBCB versus VATS-SLB were the frequency of adverse events (excluding pneumothorax) (2 % versus 13.3 % [p<0.0001]), mean length of hospital stay (2.6 days [range 0–17] versus 6.1 [range 3–48] [p<0.0001]), and mortality (0.3 % versus 2.7 % [p=0.045]), respectively.4
Conclusion: a multidisciplinary proposal on the role of cryobiopsy in the diagnosis of idiopathic pulmonary fibrosisTwo alternatives for the diagnosis of IPF in cases that require lung biopsy are currently available. Although no adequate studies comparing both techniques have yet been conducted, and despite the fact that favorable outcomes have been demonstrated for each method, our impression is that both procedures have distinct characteristics that make them more or less suitable, depending on each clinical situation. The challenge, therefore, is not to decide between 2 competing techniques in the diagnosis of patients with IPF, but to be able to recognize which is best suited to a particular situation.
Table 3 lists some circumstances that can assist in decision-making. Factors such as comorbidities, lung function, the experience of the bronchoscopy team and the pathologist, the location and extension of the radiological pattern, and the cost of the technique should be carefully assessed. The diagnostic delay must also be evaluated, since each additional procedure in the algorithm increases time to diagnosis. This proposed algorithm must be validated in prospective studies to demonstrate its clinical usefulness.
Factors to consider in each patient and site when deciding between transbronchial cryobiopsy and surgical lung biopsy.
| Cryobiopsy | Surgical lung biopsy | |
|---|---|---|
| Pros | Experience of bronchoscopy team | Experience of surgical team |
| Low comorbidity of the technique | Diagnostic yield | |
| Cost | Macroscopic visualization of the area to be biopsied | |
| Site of HRCT findings: central | Site of HRCT findings: peripheral | |
| Probable UIP pattern in context of high probability of IPF | Probable UIP pattern in context of low probability of IPF | |
| Cons | Risk of pneumothorax (severe emphysema, lesion in upper lobes) | Patient- and procedure-related comorbidities |
| Risk of hemorrhage (associated with pulmonary hypertension) | Diminished lung function values | |
| Low radiological representation | Time on procedure waiting list | |
| Pathologist’s experience in the TBCB interpretation | Cost |
HRCT: high-resolution computed tomography; IPF: idiopathic pulmonary fibrosis; TBCB: transbronchial cryobiopsy; UIP: usual interstitial pneumonia.
On the basis of all these variables, as shown in Fig. 1, each site must evaluate on a case-by-case basis which of the 2 procedures offers a better risk-benefit ratio for obtaining a histological diagnosis. Additionally, of course, studies are needed to compare the diagnostic yield and safety of SLB versus TBCB in IPF.
AuthorshipDC and ASF organized the debate and were responsible for proposing the publication of this article. DC, ASF and AT designed the manuscript. All authors contributed to the publication and have reviewed the version submitted for publication.
Conflict of interestsDC has received personal honoraria and non-financial support from Boehringer-Ingelheim and Roche, as well as honoraria from Bristol-Myers Squibb, unrelated to this manuscript; JS has received grants and personal honoraria from Boehringer-Ingelheim and Roche, and personal honoraria from Menarini, Glaxo, Novartis and Chiesi, unrelated to this manuscript. MM-M has received grants and personal honoraria from Boehringer-Ingelheim, Esteve-Teijin Healthcare, and Roche, personal honoraria from Chiesi, and grants from GSK, unrelated to this manuscript; the remaining authors declare no conflict of interest with respect to this manuscript.
This manuscript is the transcript of a debate session sponsored by Boehringer-Ingelheim.
Please cite this article as: Castillo D. et al. Propuesta multidisciplinar respecto al algoritmo diagnóstico de la fibrosis pulmonar idiopática: papel de la criobiopsia transbronquial. Arch Bronconeumol. 2020;56:99–105.