This document on COPD from the Latin American Chest Association (ALAT-2019) uses PICO methodology to analyze new evidence on inhaled medication and answer clinical questions. The following key points emerged from this analysis: 1) evidence is lacking on the comparison of short-acting vs. long-acting bronchodilators in patients with mild COPD; patients with moderate-to-severe COPD obtain greater benefit from long-acting bronchodilators; 2) the benefits of monotherapy with long-acting antimuscarinic agents (LAMA) and combined therapy with long-acting β2-agonists and inhaled corticosteroids (LABA/ICS) are similar, although the latter is associated with a greater risk of pneumonia; 3) LABA/LAMA offer greater benefits in terms of lung function and risk of exacerbation than LABA/ICS (the latter involve an increased risk of pneumonia), 4) LAMA/LABA/ICS have greater therapeutic benefits than LABA/LAMA on the risk of moderate-severe exacerbations. With regard to the role of eosinophils in guiding the use of ICS, ICS withdrawal must be considered when the initial indication was wrong or no response is elicited, in patients with side effects such as pneumonia, and in patients with a low risk of exacerbation and an eosinophil blood count of < 300 cells/μl. All this evidence, categorized according to the severity of the obstruction, symptoms, and risk of exacerbations, has been used to generate an algorithm for the use of inhaled medication in COPD.
Este documento sobre EPOC de la Asociación Latinoamericana de Tórax (ALAT)-2019 analiza las nuevas evidencias de medicación inhalada utilizando la metodología de preguntas clínicas en formato PICO. Surgen de este análisis los siguientes puntos claves: 1) No hay evidencia que compare el uso de broncodilatadores de acción corta vs. larga en pacientes con EPOC leve; en aquellos con EPOC moderada-grave existe mayor beneficio de los broncodilatadores de acción larga, 2) beneficios similares de la monoterapia con antimuscarínicos de acción prolongada (LAMA) y la terapia combinada β2-agonistas de acción larga/corticosteroides inhalados (LABA/CIS), asociada esta última a mayor riesgo de neumonía 3) mayores beneficios del LABA/LAMA en función pulmonar y riesgo de exacerbación vs. LABA/CIS (esta última con mayor riesgo de neumonía), 4) mayores beneficios de la terapia LAMA/LABA/CIS comparada con LABA/LAMA sobre el riesgo de exacerbaciones moderadas-severas. En relación al rol de los eosinófilos para guiar el uso de CIS: debe considerarse su retiro cuando la indicación inicial fue errada o sin respuesta, en pacientes con efectos secundarios, como neumonía y en aquellos con bajo riesgo de exacerbación con recuento de eosinófilos en sangre <300cels/μl. Incorporando estas evidencias según la gravedad de la obstrucción, síntomas y riesgo de exacerbaciones, se genera un algoritmo para el uso de medicación inhalada en la EPOC.
The different forms of obstructive pulmonary disease (COPD) require individualized treatment plans (precision medicine). Recent studies provide evidence of the benefits of different combinations of drugs that can impact on therapeutic regimens.1–5
In 2014, the Latin American Chest Association (ALAT) published a document on COPD using clinical questions in PICO format.6 In this document, we aim to update the information on inhaled medicines by analyzing the new evidence using the same methodology. We have focused on inhaled medications because these drugs form the basis of the pharmacological treatment of COPD. Major changes and controversies are emerging in this area, regardless of the availability of controlled clinical studies. This document is intended for clinicians, particularly respiratory specialists and other professionals involved in the care and management of patients with COPD.
MethodologyMedical specialists were invited by the COPD section of ALAT to participate in the development of this document. The working group discussed controversies in inhaled medications in COPD in 3 in-person meetings and by teleconference. New clinical questions were formulated on areas of controversy in inhaled medication, and this evidence was incorporated in a proposal for the use of this therapy. The 5 clinical questions discussed in this document were selected by consensus.
A more extensive description of the methodology, covering the formulation of clinical questions in PICO format, search strategy, eligibility criteria, critical analysis, and formulation of recommendations, can be found in Appendix B supplementary material of this document and in a prior publication.6Tables 1 and 2 describe the search strategies (Trip Database and MeSH terms), and number and type of selected studies.
Search strategy (Trip Database and MeSH terms key words).
| Clinical question | PICO question | Search strategy with MeSH terms |
|---|---|---|
| Are long-acting bronchodilators (LABA or LAMA) more effective than short-acting bronchodilators (SABA or SAMA) in patients with mild COPD? | P=COPD or Chronic Obstructive Pulmonary DiseaseI = LABA or LAMAC=SABA or SAMAO = Ø | a) (((((``pulmonary disease, chronic obstructive”[MeSH Terms] OR copd OR chronic obstructive pulmonary disease OR coad OR chronic obstructive airway disease OR chronic obstructive lung disease OR airflow obstruction, chronic OR airflow obstructions, chronic OR chronic airflow obstructions OR chronic airflow obstruction))))) AND (``Albuterol”[MeSH] OR salbutamol OR 2-t-Butylamino-1- AND (4-hydroxy-3-hydroxy-3-hydroxymethyl) AND phenylethanol OR ventolin OR sultanov OR albuterol sulfate OR prove it)b) (((((``pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction))))) AND (``Ipratropium”[MeSH] OR atrovent OR ALovent) |
| Does the combination of LABA+ICS provide greater benefits than monotherapy with LAMA or dual bronchodilator therapy with LABA+LAMA? | P=COPD or Chronic Obstructive Pulmonary DiseaseI = LABA+ICSC=LAMA or LAMA+LABAO = Ø | ((((((``pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction))))) AND ``Bronchodilator Agents”[MeSH]) AND Inhale* corticosteroids* |
| Does the combination of LABA+LAMA+ICS (triple therapy) provide greater benefits compared with LAMA monotherapy, combination therapy (LABA/ICS) or dual bronchodilator therapy (LABA/LAMA) in patients with COPD? | P=COPD or Chronic Obstructive Pulmonary DiseaseI = LABA+LAMA+ICSC=LAMA or LABA+ICs or LAMA+LABAO = Ø | (((((``pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction))))) AND Triple Therapy |
| Which COPD patients benefit from the use of ICS in the reduction of exacerbations? | P=COPD or Chronic Obstructive Pulmonary DiseaseI = inhaled corticosteroidsC = ØO=exacerbation* OR mortality | ((((((``pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction)))) AND Inhale* corticosteroid*) |
| In which patients can ICS be safely withdrawn? | P=COPD or Chronic Obstructive Pulmonary DiseaseI = ((withdrawal of ICS) OR (withdrawal of corticoid*))C = ØO = Ø | ((((``pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction)))) AND Withdra* AND Inhale* AND corticosteroid* |
COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long-acting β2-agonists; LAMA: long-acting antimuscarinic agents; RCT: randomized controlled trials; SABA: short-acting β-agonist; SAMA: short-acting muscarinic antagonist.
Number and type of studies selected to answer clinical questions.
| Clinical question | Total references selected | Total references retrieved from the Trip Database | Total references retrieved from MeSH | Total references selected to answer the question | Type of studies selected |
|---|---|---|---|---|---|
| Are long-acting bronchodilators (LABA or LAMA) more effective than short-acting bronchodilators (SABA or SAMA) in patients with mild COPD? | 485 | 20 | 465 | 2 | 2 systematic reviews12,13 |
| Does the combination of LABA plus ICS provide greater benefits than monotherapy with LAMA or dual bronchodilator therapy with LABA+LAMA? | 238 | 8 | 230 | 5 | 5 systematic reviews23–27 |
| Does the combination of LABA+LAMA+CIS (triple therapy) provide greater benefits compared with LAMA monotherapy, combination therapy (LABA/ICS) or dual bronchodilator therapy (LABA/LAMA) in patients with COPD? | 193 | 12 | 181 | 7 | 2 systematic reviews30,315 RCTs3,4,32–34 |
| Which COPD patients benefit from the use of ICS in the reduction of exacerbations? | 338 | 92 | 246 | 5 | 3 systematic reviews26,36,372 RCTs3,38 |
| In which patients can ICS be safely withdrawn? | 588 | 341 | 247 | 3 | 1 meta-analysis452 RCTs46,47 |
COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long-acting β2-agonists; LAMA: long-acting antimuscarinic agents; RCT: randomized controlled trials; SABA: short-acting β-agonist; SAMA: short-acting muscarinic antagonist.
Using a cutoff date of September 2018, we rated publications in Spanish, Portuguese, and English according to the ACCP grading system, and classified the recommendation as strong or weak, according to risk, benefit, and burden ratios, and occasionally, cost. The quality of evidence was classed as high, moderate, or low, depending on the design of the study, consistency of results, and clarity of the evidence to answer the clinical question.
Bronchodilator monotherapyQuestion: are long-acting bronchodilators (LABA or LAMA) more effective than short-acting bronchodilators (SABA or SAMA) in patients with mild chronic obstructive pulmonary disease?RationaleAround 70 % of patients with COPD have mild-moderate airflow obstruction (FEV1 ≥ 50 %), with few respiratory symptoms.7–10 Information on inhaled medication in the initial stages or in mild disease is limited. Only 2 randomized controlled trials (RCT) in patients with mild-moderate obstruction have assessed the benefits of treatment with long-acting bronchodilators (BD) vs. placebo: one with tiotropium11 and another with the combination of long-acting ®2-agonists+inhaled corticosteroids (LABA/ICS).12 Tiotropium showed improvement in forced expiratory volume in 1s (FEV1), quality of life, frequency of exacerbations, and lung function decline.11 In mild COPD, the use of any BD is usually recommended, so it would be interesting to analyze whether a long-acting BD instead of a short-acting BD is justified in these patients.
Search outcomeA total of 485 references (MeSH: 465; Trip Database: 20) were retrieved, and 2 systematic reviews were selected to answer the question.13,14
Quality of evidenceIn terms of efficacy, a systematic review comparing tiotropium vs. ipratropium (SAMA) in patients with moderate-severe obstruction shows greater benefit for tiotropium in lung function (increased FEV1: 109ml; 95 % CI: 80−137ml), quality of life (St. George's Respiratory Questionnaire [SGRQ] difference: −3.3; 95 % CI: 0.97–5.63), fewer hospitalizations (OR: 0.34; 95 % CI: 0.15-0.76) and exacerbations (OR: 0.71; 95 % CI: 0.52–0.95).13 Another systematic review comparing ipratropium and LABAs14 in patients with moderate-severe obstruction showed greater benefits for salmeterol in FEV1 (60ml; 95 % CI: 110−0ml) and morning peak flow (−10.96 lit/min; 95 % CI: −16.09 to −5.83), with no difference in quality of life, exacerbations, rescue medication, exercise capacity, or symptoms. The use of formoterol compared with ipratropium seems to show improvement in morning peak flow with no difference in FEV1, quality of life, dyspnea, or exercise capacity.
In terms of safety, the study that compared ipratropium vs. tiotropium reported fewer serious adverse events (OR: 0.50; 95 % CI: 0.34–0.73) and disease events with tiotropium (OR: 0.59; 95 % CI: 0.41–0.85), and no differences in mortality.13 No comparative studies of long-acting BD monotherapy vs. short-acting ®2-agonists (SABA), or comparative studies between short-acting vs. long-acting BD in patients with mild obstruction were retrieved.
Conclusions and recommendationsThere is no available evidence that compares the use of short and long-acting BDs in COPD patients with mild obstruction. The comparative studies retrieved in the search and the evidence selected involves patients with moderate-severe obstruction. These show that, in terms of efficacy, tiotropium bromide+LABA compared with ipratropium has greater benefits in lung function. Tiotropium also showed greater benefits in dyspnea, exacerbations, and quality of life, and a better safety profile.
The only recommendation that can be drawn from these findings is that LABA or tiotropium should be used in preference to ipratropium in COPD patients with moderate-severe obstruction in terms of dyspnea, quality of life, and lung function, and tiotropium bromide in preference to ipratropium in terms of improved exacerbation and hospitalization rates.
Combined therapies (LABA/ICS, LABA/LAMA and LABA/LAMA/ICS)Question: does the combination of LABA+ICS provide greater benefits than monotherapy with LAMA or dual bronchodilator therapy with LABA+LAMA?RationaleLAMA monotherapy offers benefits in dyspnea, quality of life, and frequency of exacerbations and hospitalizations.15–18 A reduction in exacerbations and improved quality of life and lung function have also been reported for LABA/ICS19 and LABA/LAMA.18,20–29 The question arises as to whether there are differences between these treatments.
Search outcomeA total of 238 references (MeSH: 230; Trip Database: 8) were retrieved, and 4 systematic reviews were selected to answer the question.30,32–34
Quality of evidenceIn terms of efficacy, a systematic review comparing fluticasone/salmeterol vs. tiotropium in patients with moderate-severe obstruction shows similar results in exacerbation and hospitalization rates and quality of life.30 However, the number of drop-outs in one of the studies was high, the groups were poorly matched, and patients were not followed up after drop-out, which limits the applicability of its results.31
Another systematic review in patients with moderate-severe obstruction showed modest improvement (without clinical relevance) with LABA/ICS (fluticasone/salmeterol) compared with tiotropium in pre-BD FEV1 (60ml), rescue medication, and quality of life (SGRQ, −2.07 units).32
A systematic review compared the effectiveness of LABA/LAMA with LABA/ICS (fluticasone/salmeterol) in patients with mostly moderate-severe COPD.33 LABA/LAMA showed greater benefits in lung function (trough FEV1 [MD 80ml]) and risk of exacerbations (OR: 0.82). There was no difference in quality of life (SGRQ total score); however, the minimum clinical difference of 4 points was achieved more frequently with LABA/LAMA than with fluticasone/salmeterol (OR: 1.25).33
Another systematic review and meta-analysis in patients with moderate-very severe COPD showed greater benefits from LABA/LAMA in lung function (trough FEV1 [MD 80ml]), risk of moderate-severe exacerbations (RR: 0.82), and use of rescue medication (-0.18 puffs/day) compared with LABA/ICS (fluticasone/salmeterol).34 There was no difference between the therapies in quality of life or severity of dyspnea.
With regard to safety, systematic reviews show an increased risk of pneumonia and serious adverse effects with fluticasone/salmeterol vs. tiotropium or LABA/LAMA.33
Conclusions and recommendationsThe efficacy of tiotropium and fluticasone/salmeterol in patients with moderate-severe COPD is similar. LABA/LAMA has greater benefits in lung function and risk of exacerbations compared with fluticasone/salmeterol. With regard to safety, there is evidence of an increased risk of pneumonia with fluticasone/salmeterol vs. tiotropium bromide+LABA/LAMA.
HIGH evidence for the use of tiotropium or LABA/ICS (fluticasone/salmeterol) in terms of dyspnea, pulmonary function, quality of life, and frequency of exacerbations and hospitalizations in patients with moderate-severe COPD.
STRONG recommendation to prefer tiotropium over fluticasone/salmeterol, due to an increased risk of pneumonia with the latter.
HIGH evidence and STRONG recommendation for the use of LABA/LAMA in preference to LABA/ICS (fluticasone/salmeterol) to improve lung function and frequency of exacerbations, with less risk of pneumonia in patients with moderate-very severe COPD.
Question: does the combination of LABA+LAMA+ICS (triple therapy) provide greater benefits compared with LAMA monotherapy, combination therapy (LABA/ICS) or dual bronchodilator therapy (LABA/LAMA) in patients with COPD?RationaleThe combination of LABA/LAMA/ICS may decrease the risk of exacerbations, hospitalizations, and healthcare costs in COPD patients with moderate-very severe obstruction.35,36 Controversy persists on the efficacy and safety of fixed-dose combination triple therapy or combining different devices (LABA/ICS+tiotropium or LABA/ICS+glycopyrronium), compared with LAMA, LAMA/ICS or LABA/LAMA.
Search outcomeA total of 193 references (MeSH: 181; Trip Database: 12), and 2 systematic reviews37,38 and 5 RCTs3,4,39–41 were selected to answer the question.
Quality of evidenceOne systematic review showed greater benefits with triple therapy (LABA/ICS+tiotropium) in hospitalizations for all causes (reduction of risk: 39 %; OR: 0.61; 95 % CI: 0.40–0.92), quality of life (SGRQ difference: −3.46; 95 % CI: −5.05 to −1.87), and lung function (pre-BD FEV1: 60ml; 95 % CI: 40−80ml at 3–6 months) compared with tiotropium in patients with moderate-severe COPD, with no differences in mortality or frequency of exacerbations.37 Similar results were reported by another systematic review and meta-analysis.38 Three RCTs in patients with FEV1 < 50 % and a history of exacerbations evaluated the efficacy and safety of a fixed-dose combination (beclomethasone dipropionate+formoterol fumarate+glycopyrronium [BDP/FF/GLY]) compared with tiotropium39; with BDP/FF41; and with indacaterol+glycopyrronium.4 BDP/FF/GLY compared with tiotropium showed greater benefits in the frequency of moderate-severe exacerbations (RR: 0.80; 95 % CI: 0.69 to −0.92), lung function (pre-BD FEV1 difference: 61ml; 95 % CI: 37−86ml), quality of life (SGRQ responders: 1.33; 95 % CI: 1.10–1.59), and reduced use of rescue medication.39 The combination showed greater benefits compared with BDP/FF in lung function at 26 weeks (FEV1 pre-dose difference: 81ml; 95 % CI: 52−109ml), a 23 % reduction in moderate-severe exacerbations (RR: 0.77; 95 % CI: 0.65-0.92), quality of life (responders) at 52 weeks (SGRQ, OR: 1.33; 95 % CI: 1.06–1.66), with no differences in dyspnea severity.41 BDP/FF/GLY compared to indacaterol/glycopyrronium showed greater benefits in the frequency of moderate-severe exacerbations (RR: 0.85; 95 % C: 0.72-0.99); there were no differences in lung function or quality of life.4
Two RCTS compared a fixed-dose combination of fluticasone furoate/umeclidinium/vilanterol (FFL/UMEC/VI) with budesonide/formoterol (BUD/FF),40 FFL/VI and UMEC/VI3 in symptomatic patients with moderate-severe obstruction and history of exacerbations. Compared to BUD/FF, the triple therapy showed greater benefits in lung function (trough FEV1: difference 171ml; 95 % CI: 148–194, in favor of FFL/UMEC/VI), quality of life (SGRQ difference: −2.2; 95 % CI: −3.5 to −1, in favor of FFL/UMEC/VI), and the frequency of moderate-severe exacerbations (35 % reduction; 95 % CI: 14 %–51 %). A subanalysis40 shows similar benefits with FFL/UMEC/VI over BUD/FF in symptomatic patients, regardless of the severity of COPD or prior treatment. One of the studies3 showed greater benefits with FFL/UMEC/VI in the frequency of moderate-severe exacerbations, compared with FFL/VI (RR: 0.85; 95 % CI: 0.80 %–0.90 %, 15 % difference) and UMEC/VI (RR: 0.75; 95 % CI: 0.70–0.81, 25 % difference), regardless of the eosinophil count in blood, although there was a greater reduction of risk in patients with eosinophils > 150 cells/μl. This combination also showed greater benefits in lung function (FFL/UMEC/VI vs. FFL/VI, trough FEV1 difference: 97ml; 95 % CI: 85−109ml, and FFL/UMEC/VI vs. UMEC/VI, trough FEV1 difference: 54ml; 95 % CI: 39−69ml) and quality of life (SGRQ, FFL/UMEC/VI vs. FFL/VI difference −1.8; 95 % CI: −2.4 to −1.1, and FFL/UMEC/VI vs. UMEC/VI −1.8; 95 % CI: −2.6 to −1.0). With regard to safety, triple therapy in different devices compared to tiotropium showed no differences in the appearance of adverse effects.37,38 BDP/FF/GLY showed an incidence of pneumonia in a small group of patients (BDP/FF/GLY 2 % vs. tiotropium 1 %).40 There were no differences in the incidence of pneumonia between BDP/FF/GLY and BDP/FF41 or indacaterol/glycopyrronium4; however, the risk of a medical diagnosis of pneumonia with FFL/UMEC/VI was higher than with UMEC/VI (HR: 1.52; 95 % CI: 1.22–1.92).3
Conclusions and recommendationsIn symptomatic COPD patients with severe-very severe obstruction and a history of exacerbations, triple therapy offers greater benefits in terms of efficacy, lung function, quality of life, and risk of exacerbations than tiotropium or LABA/ICS. Triple therapy compared with LABA/LAMA shows greater benefits in the risk of moderate-severe exacerbations. In comparison with FFL/VI or UMEC/VI, the FFL/UMEC/VI combination shows greater benefits in the frequency of moderate-severe exacerbations, regardless of eosinophil blood counts; although the benefit is greater in patients with > 150 cells/μl. The risk of pneumonia is greater in treatments containing ICS.
HIGH evidence and STRONG recommendation for the use of triple therapy in symptomatic COPD patients with severe-very severe obstruction and risk of exacerbations to improve lung function and quality of life and decrease the risk of exacerbations.
Question: which COPD patients benefit from the use of ICS in the reduction of exacerbations?RationaleThe use of ICS alone or in combination with LABA has shown benefits in COPD patients, including lower exacerbation rates and health status decline.42,43
These outcomes should be analyzed in terms of the risk/benefit ratio, in particular the risk of pneumonia associated with ICS.
A need is emerging to define the subgroup of patients with COPD who benefit most from ICS, focused on reducing the risk of exacerbation.
Search outcomeA total of 338 references (MeSH: 246; Trip Database: 92), and 3 systematic reviews33,44,45 and 2 RCTs3,46 were selected to answer the question.
Quality of evidenceA systematic review comparing the efficacy of any dose or type of ICS with placebo in patients with moderate-severe COPD showed that ICS reduced the rate of exacerbations (-0.26 exacerbations per patient/year, 95 % CI: −0.37 to −0.14).44 Another systematic review comparing the effectiveness of LABA/ICS (mainly fluticasone/salmeterol) with ICS monotherapy in patients with mild-severe COPD showed a reduction in the frequency of exacerbation with LABA/ICS (RR: 0.91; 95 % CI: 0.85–0.97).45 Two RCTs with fluticasone/salmeterol included in this review showed that exacerbations requiring oral steroids were reduced with fluticasone/salmeterol, and another found no difference in the rate of hospitalization.45 A systematic review comparing the effectiveness of LABA/LAMA with LABA/ICS (fluticasone/salmeterol) in patients with mostly moderate-severe COPD showed greater benefits with LABA/LAMA in the risk of exacerbations (OR: 0.82; 95 % CI: 0.70–0.96). The studies included in the analysis were heterogeneous, and included an observation period of less than 1year. Most included patients with moderate-severe COPD, with no recent exacerbations.33
An RCT that compared FFL/UMEC/VI with FFL/VI and UMEC/VI3 in patients with moderate-very severe obstruction and a history of exacerbations showed greater benefits with FFL/UMEC/VI in frequency of moderate-severe exacerbations, compared with FFL/VI (RR: 0.85; 95 % CI: 0.80 %–0.90 %, 15 % difference) and UMEC/VI (RR: 0.75; 95 % CI: 0.70–0.81, 25 % difference), regardless of the eosinophil count in blood, although there was a greater reduction of risk in patients with a eosinophil count > 150 cells/μl.
Another RCT evaluated the effect of intensifying LABA/ICS therapy (budesonide/formoterol) on the exacerbation rate in moderate-severe patients at the onset of upper respiratory tract infection.46 The incidence of exacerbations in the budesonide/formoterol and placebo groups was similar (14.6 vs. 16.2 %; HR: 0.77; 95 % CI: 0,46–1,33), although the risk of severe exacerbations was reduced by 72 % (HR: 0.28; 95 % CI: 0,11–0,74) with intensified therapy. A significantly reduced risk of exacerbation was observed in the subgroup of patients with more severe disease.
In terms of safety, evidence suggests that treatments that include ICS are associated with more frequent serious adverse effects, in particular, an increased risk of pneumonia.3,33,44
Conclusions and recommendationsIn terms of efficacy, the use of long-term ICS shows a benefit in the risk of exacerbations in patients with moderate-severe COPD, which is greater in individuals with elevated eosinophils in blood. This benefit must be weighed up against the increased risk of pneumonia.
Moderate evidence and strong recommendation for the use of ICS in patients with moderate-severe COPD with a history of exacerbation and elevated eosinophils in blood, in terms of a reduced risk of exacerbations.
Question: in which patients can ICS be safely withdrawn?RationaleOveruse of ICS in COPD is a common practice,47–49 even though these medications are usually reserved for patients at high risk of exacerbations (one third of the total population).50,51 The long-term use of ICS is associated with an increased risk of adverse events, particularly pneumonia.42,52 Patients who are unlikely to benefit and in whom discontinuation is safe must be identified.
Search outcomeA total of 588 references (MeSH: 247; Trip Database: 341) were retrieved, and 1 meta-analysis53 and 2 RCTs54,55 were selected to answer the question.
Quality of evidenceA meta-analysis that includes RCTs and real-world observational studies in patients with moderate-severe obstruction showed no increase in the overall risk of exacerbations after ICS withdrawal (OR: 1.03; 95 % CI: 0.95–1.12; p>0.05). However, the risk of moderate-severe exacerbation increased (2.4 % and 33.6 %, respectively), and the time to first exacerbation was shorter (p<0.05) in patients who discontinued ICS. ICS withdrawal was also associated with lung function decline (FEV1: −30ml) and reduced quality of life (+1,24 SGRQ units), without reaching a minimally significant clinical difference.53
An RCT evaluated the efficacy and safety of abrupt withdrawal of ICS from long-term triple therapy in COPD patients who were frequent exacerbators with FEV1 between 40 %–80 %.54 Patients were randomized to continue fluticasone/salmeterol+tiotropium or to switch to indacaterol/glycopyrronium. No differences were observed between groups in the frequency of moderate-severe exacerbations (0.52 vs. 0.48) (RR: 1.08; 95 % CI: 0.83–1.40) or the time to the first moderate-severe exacerbation (HR: 1.11; 95 % CI: 0.85–1.46).54 The withdrawal of ICS was associated with a slight reduction in trough FEV1: −26ml (95 % CI: −53 to 1ml).54 Patients with a blood eosinophil count ≥ 300 cells/μl presented greater deterioration in lung function and an increased risk of exacerbation (RR: 1.86; 95 % CI: 1.06–3.29) Adverse events were similar between the groups.54 Another RCT assessed changes in airway inflammation after fluticasone was withdrawn in patients with moderate-severe COPD who received this medication long term.55 The interruption of fluticasone induced an increase in bronchial T cells, mast cells, and several types of cells in sputum (relapse in the production of inflammatory cells), which was accompanied by further lung function decline.55 The results suggest that airway inflammation is suppressed during treatment with fluticasone, but the nonsteroidal anti-inflammatory effects are not maintained after withdrawal.55
Conclusions and recommendationsICS can be withdrawn abruptly in COPD patients with a low risk of exacerbation, moderate-severe obstruction, and blood eosinophil count < 300 cells/μl without increasing the risk of exacerbation or affecting lung function. ICS should not be withdrawn in patients at high risk of exacerbation and moderate-severe obstruction, due to an increased risk of exacerbation and lung function decline.
Moderate evidence and strong recommendation for withdrawal of ICS in COPD patients with a low risk of exacerbations, moderate-severe obstruction, and eosinophil blood count < 300 cells/μl.
Incorporation of new evidenceThe treatment of COPD should be individualized according to disease severity and drug availability. General measures and prevention (education, smoking cessation, vaccination, and physical activity, among others), and pharmacological and non-pharmacological treatments should be taken into account.
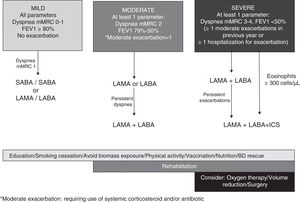
In view of all the evidence analyzed, we propose a scheme with progressive inhaled medication according to COPD severity (dyspnea, obstruction, or exacerbations) that can be modified according to clinical response (Fig. 1).
BD monotherapy is recommended in patients with mild disease (all criteria: dyspnea mMRC grade 1, FEV1 ≥ 80 % post-BD).
The efficacy and safety of LAMA vs. LABA monotherapy were analyzed in an earlier study.6 The evidence shows that in terms of efficacy, tiotropium and LABAs have similar benefits in dyspnea, lung function, and quality of life, but tiotropium is more effective in reducing the frequency of exacerbations. On the basis of the evidence analyzed, patients with moderate disease (with at least 1 parameter of severity: dyspnea mMRC grade 2, FEV1 79 %−50 %, an exacerbation without hospitalization in the preceding year) should start bronchodilator monotherapy with LAMA or LABA, instead of LABA/ICS in view of the increased risk of pneumonia associated with ICS. LAMA/LABA can be escalated, according to response. The LABA/ICS combination is recommended in patients with asthma or a medical diagnosis of asthma before the age of 40 years (asthma−COPD overlap).56
Patients with severe disease (at least 1 severity parameter: mMRC dyspnea grade 3–4, FEV1 <50 %, ≥ 2 exacerbations in the last year, or ≥ 1 hospitalization for exacerbation) should start treatment with LAMA/LABA, except individuals with an eosinophil blood count ≥ 300 cells/μl, who should receive LABA/LAMA/ICS, given the benefits of triple therapy in the risk of exacerbations and lung function decline.54 Escalation to LABA/LAMA/ICS is indicated in patients who started LAMA/LABA, but who persist with exacerbations regardless of their eosinophil count.3 ICS withdrawal must be considered in certain circumstances: when the initial indication was incorrect or no response is elicited; in patients with side effects such as pneumonia; and in patients with a low risk of exacerbation and a serum eosinophil count of <300 cells/μl.54 The prophylactic use of azithromycin or roflumilast can be useful as additional therapy in reducing the number of exacerbations in severe patients.57,58
ConclusionsThis ALAT 2019 statement provides an overview of treatment with inhaled medication in chronic obstructive pulmonary disease (COPD), incorporating the evidence analyzed using PICO methodology, according to severity of the obstruction, symptoms, and risk of exacerbations.
Conflict of interestsAugustín Acuña and Ephraim Sanchez declare that they have received professional honoraria for the development and implementation of methodological aspects of this paper from ITSalud/Medsolid. All other authors declare no real or perceived conflict of interest.
FundingThis study was funded by AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. The sponsors did not play any part in the study and did not participate at any stage of the development of these guidelines. None of the authors was paid for their participation in the preparation of this update.
Please cite this article as: Montes de Oca M et al. Incorporando nuevas evidencias sobre medicamentos inhalados en la EPOC. Asociación Latinoamericana de Tórax (ALAT) 2019. Arch Bronconeumol. 2020;56:106–113.