Lung transplantation (LT) is the only effective treatment available for some patients with end-stage lung disease. Despite improvements in surgical and medical care, LT is associated with considerable morbidity and mortality. Primary graft dysfunction (PGD) is a syndrome of acute lung injury occurring in the early stage post-lung transplantation.1 PGD is the main cause of mortality in the first month of transplant and the second cause during the first year.2
Statins, 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors, have been described as the most effective class of drugs to reduce serum cholesterol levels.3 In recent years, it has also been reported that statins have a variety of immunomodulatory and antiinflamatory effects unrelated to their cholesterol-lowering function3–6 and could have a positive impact on LT recipients. A few studies have reported that dyslipidaemia is an independent risk factor for PGD7 and that perioperative use of statins is independently associated with reduced risk for PGD.8
We report a retrospective, multicenter cohort study aiming to evaluate the impact of recipient preoperative statin therapy on the development of PGD on adult patients undergoing first time uni- or bilateral lung transplantation from brain death donors at four transplant centres in Spain, (January 2015 to December 2017). Comparison was made between groups according to whether the recipient had previously used statins (rSG) or not (rNSG) as dyslipidemia treatment. All centres followed recipient acceptance criteria established by the Organizacion Nacional de Trasplantes (ONT).9 PGD incidence and its severity, as well as 30, 90 and 360-day survival rate were analyzed. International Society for Heart and Lung Transplantation (ISHLT) Working Group criteria for the definition and severity grading of PGD were used.1
Categorical variables are expressed as percentages. Quantitative data are presented as mean and standard deviation (SD) if normally distributed or median if otherwise. The paired t test or the U-Mann–Whitney test and the Chi-square test or Fisher's exact test were used to compare continuous and categorical variables, respectively. Propensity score was calculated through a multivariate analysis including those variables that were found significantly different (p<0.05) between SG and NSG in the univariate analysis. An univariate analysis was performed in those patients developing PGD, trying to identify the factors impacting on its severity (PGD 3 vs. PGD 1–2). Logistic regression model for PGD severity was built including those variables with p≤0.1 in the univariate analysis.
A total of 474 consecutive first single and double adult LT procured from 387 brain death donors were included. One hundred and ten recipients (SG, 23.2%) were under statins treatment before transplantation (Table 1).
Donor and recipients’ characteristics according to statin pretransplant treatment in the recipient.
| Variable | No statins (N=364) | Statins (N=110) | p |
|---|---|---|---|
| Recipient | |||
| Clinical data | |||
| Age, years, mean (SD) | 53 (12) | 60 (7) | <0.001 |
| Gender (%) | 0.025 | ||
| Male | 222 (61.0) | 80 (72.7) | |
| Female | 142 (39.0) | 30 (27.3) | |
| BMI, kg/m2, mean, (SD) | 24.8 (4.2) | 26.3 (3.8) | 0.001 |
| Disease | 0.005 | ||
| Restrictive | 170 (46.7) | 58 (52.7) | |
| COPD | 123 (33.8) | 45 (40.9) | |
| CF/BC | 39 (10.7) | 1 (0.9) | |
| PPH | 18 (4.9) | 1 (0.9) | |
| Other | 14 (3.8) | 5 (4.5) | |
| Surgical data | |||
| Blood transfusion (%) | 0.454 | ||
| Yes | 134 (47.9) | 39 (43.3) | |
| No | 146 (52.1) | 51 (56.7) | |
| Vasoactive drugs (%) | <0.001 | ||
| Yes | 224 (77.8) | 53 (58.9) | |
| No | 64 (22.2) | 37 (41.1) | |
| CPB (%) | 0.159 | ||
| Yes | 60 (20.7) | 25 (27.8) | |
| No | 230 (79.3) | 65 (72.2) | |
| Ischaemic time first graft, min, mean (SD) | 269 (72) | 275 (69) | 0.285 |
| Ischaemic time second graft,min, mean (SD) | 393 (322) | 362 (98) | 0.695 |
| Donora | |||
| Clinical data | |||
| Age, years, mean (SD) | 53 (13) | 56 (13) | 0.008 |
| Gender (%) | 0.112 | ||
| Male | 177 (48.6) | 44 (40.0) | |
| Female | 187 (51.4) | 66 (60.0) | |
| BMI, kg/m2, mean, (SD) | 25.9 (4.1) | 26.4 (4.2) | 0.205 |
| PaO2, mmHg, mean (SD) | 444 (79) | 438 (84) | 0.507 |
| Smoking | 0.305 | ||
| No smoking (%) | 203 (60.8) | 61 (61.0) | |
| Smoking (%) | 91 (27.2) | 32 (32.0) | |
| Former smoking (%) | 40 (12.0) | 7 (7.0) | |
| Surgical data | |||
| Blood transfusion (%) | 1.000 | ||
| Yes | 3 (5.4) | 1 (3.4%) | |
| No | 53 (94.6) | 28 (96.6) | |
| Vasoactive drugs (%) | 0.514 | ||
| Yes | 74 (85.1) | 41 (89.1) | |
| No | 13 (14.9) | 5 (10.9) | |
BMI: body mass index; CPB: cardiopulmonary bypass; CF/BC: fibrosis/bronchiectasis; PPH: primary pulmonary hypertension.
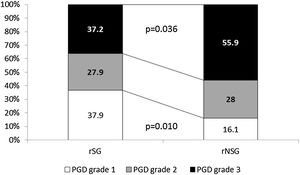
Global PGD incidence was 34%, with no significant difference between groups. However a significantly lower incidence of grade 3 PGD (37.2% vs. 56.9%, p=0.036) (Fig. 1), as well as better 30d survival (100% vs. 96%, p=0.028) was observed in rSG. No differences in mortality at 60 (94 vs 94, p=0.91) and 90 (85 vs 86, p=0.75) days were found between groups.
The influence of statin treatment on lung donors before retrieval was analyzed with no differences observed between the group taking statins and the one not taking in incidence (38.7% vs. 36.2%, p=0.69) and severity of PGD (PGD1 6.9 vs. 22.1, PGD2 34.5 vs. 29.2, PGD3 58.6 vs. 48.7; p=0.176)
Patients developing grade 3 PGD had higher body mass index (BMI) than those developing milder PGD grades. Similarly, more recipients developing grade 3 PGD had not received pre-transplant statin therapy, required hemoderivate transfusion and underwent cardiopulmonary bypass during surgery than those patients developing PGD grade 1 or 2. We analyzed the impact of recipient's disease in PGD's incidence between PGD grade 1–2 and 3 and no statistical differences were seen (COPD 49.1% vs.50.9%, restrictive 50% vs. 50%, Cystic Fibrosis/Bronchiectasis 53.8% vs. 46.2%, primary pulmonary hypertension 60% vs. 40% other 0% vs. 100%; p=0.347)
Multivariate analysis identified the need for hemoderivate transfusion in the recipient during surgery as a factor associated with higher severity of PGD (OR 4.65, 1.27–17.04 p=0.02). Similarly the use of statins showed a trend towards statistical significance(OR 2.28, 0.69–7.57 p=0.17).
This is, to our knowledge, the first multicentric study analyzing the effect of preoperative use of statins in LT recipients and their immediate outcomes. Several authors have evaluated the impact of statins on LT outcome, however these studies were performed in a single centre or evaluated the impact of recipient pretransplant dyslipidaemia7 or perioperative treatment with statins8 on transplant outcomes.
We observed a PGD incidence of 34%, similar to data reported in the literature that ranges from 30% to 50% early after transplant.10 In the present study, recipient's preoperative statin treatment was associated with a significant decrease in the incidence of severe PGD. Raphael et al.8 reported a decreased incidence of PGD grade 3 in those recipients using statins perioperatively (34.8% vs. 57.9%, p=0.001). However, the study was performed in a retrospective analysis of 266 patients undegoing LT in a single centre.
The presence of PGD has been shown to be associated with higher postoperative 30-day,11 and 90-day mortality12 after transplant. Kreisel et al.,13 described a 1-year survival of 72.8% on those lung recipients developing PGD. We report higher 30-day, 90-day and 1-year survival in those patients developing PGD grade 3 than the one reported by Kreisel. Moreover, in our study recipients in the SG had a significantly decreased 30-day mortality when compared to those not taking statins.
The antioxidant action of statins has been proposed as a potential mechanism by which these agents may improve endothelial function against oxidative stress,14 reported as an important factor in the pathogenesis of PGD.15 Recent data also reveals the anti-inflammatory effect of statins due to their potent inhibitory action against the induction of several proinflammatory cytokines.3 Murphy et al.5 reported the ability of simvastatin to attenuate the ex vivo production of epithelium-derived mediators of neutrophilic airway inflammation. Similarly the identification of several mechanisms through which statins may decrease the recruitment of monocytes and T cells and inhibit T cell activation and proliferation has prompted the view that statins could be beneficial in organ transplant recipients.14 Finally statins inhibit the transcription of major histocompatibility complex class II molecules16 and up-regulate T-cells, which is associated with improved early graft function after LT in mice and in humans.17
In conclusion, our results show that preoperative statin therapy in LT recipients might decrease the incidence of severe PGD and improve 30d survival. Future adequately powered prospective studies are needed to determine the real role of statins in the lung transplant procedure, as well as the identification of type and optimal dosage of statins that will better help to decrease the risk of PGD development.
Disclosure statementNone of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
Writing support was provided by Fidelma Greaves.