Risk stratification remains central to guiding therapy in pulmonary embolism (PE). European Society of Cardiology (ESC) Guidelines recommend systemic thrombolysis (ST) in high-risk (HR) PE and in selected intermediate-high risk (IHR) PE patients who clinically deteriorate despite anticoagulation [1]. However, only about 30% of patients with HR-PE in real-world practice receive ST [2–4]. Emerging evidence suggests that certain clinical parameters, such as elevated serum lactate, borderline hypotension (systolic blood pressure [SBP] 90–100mmHg), or persistent tachycardia, may identify a subgroup of IHR-PE patients at risk of adverse outcomes, not adequately captured by the ESC classification [5–8]. In this context, the RIsk claSsification Adapting SCAI shock stages to right ventricular failure in Pulmonary Embolism (RISA-PE) was proposed. This 5-stage model (A–E) mirrors the SCAI classification for left ventricular shock and aims to more precisely define the progression of right ventricular (RV) failure in patients with IHR- and HR-PE. In a prior cohort of patients treated with catheter-directed intervention (CDI), RISA-PE showed a stepwise increase in in-hospital mortality and superior discrimination compared to the ESC classification [9]. The objective of the present study was to validate the prognostic performance of the RISA-PE classification in a non-interventional cohort of IHR- and HR-PE patients.
This observational, multicenter study included consecutive patients between January 2009 and March 2024 with IHR- and HR-PE, as defined by the ESC Guidelines, who were managed with medical treatment (anticoagulation and/or ST). The study retrospectively combined patients from three hospitals in Spain with two ongoing, prospective PE registries and patients from the PROTECT prospective international multicenter cohort who fulfilled the inclusion/exclusion criteria. Inclusion criteria were: adult patients (≥18 years) with a confirmed diagnosis of acute PE, classified as HR or IHR according to ESC guidelines [1], and admitted primarily due to this condition. Exclusion criteria were: subacute PE (symptom duration>7 days), treatment with percutaneous intervention or surgery, and PE cases with insufficient data for risk stratification and/or in-hospital outcomes. Details on ethical approvals are provided in the Supplementary material. The primary outcome of the present study was in-hospital all-cause mortality. The RISA-PE classification is presented in Supplementary Table 1. In summary, the ESC IHR category is further stratified in stage A (normal lactate and shock index [heart rate/systolic blood pressure]<1) and stage B (serum lactate ≥2mmol/L or shock index ≥1, or both). Those patients with HR-PE were reclassified according to the clinical presentation in stage C (persistent hypotension without established shock), D (obstructive shock) and E (cardiac arrest). Investigators assigned the clinical subgroup within HR-PE according to the ESC Guidelines. The first lactate measurement upon patient arrival to the hospital, irrespective of whether it was an arterial or venous sample, was used for stratification. Regarding the statistical analysis, all-cause mortality through RISA-PE stages was analyzed with a logistic regression model, and a post-estimation test of the linear hypothesis across stages was conducted using coefficients of orthogonal polynomials, to obtain the linear trend's p-value. Discriminative power of the RISA-PE and the ESC classifications in predicting in-hospital all-cause mortality was assessed using the area under the ROC curve (AUC) for each model. The calibration of in-hospital mortality prediction was assessed using the Brier score for both classifications. In addition, sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios for in-hospital mortality were calculated using a binary cut-off of A vs B-E in the RISA-PE classification. Finally, to assess reclassification, the integrated discrimination improvement (IDI) and net reclassification index (NRI) were calculated. Further statistical details are provided in Supplementary material.
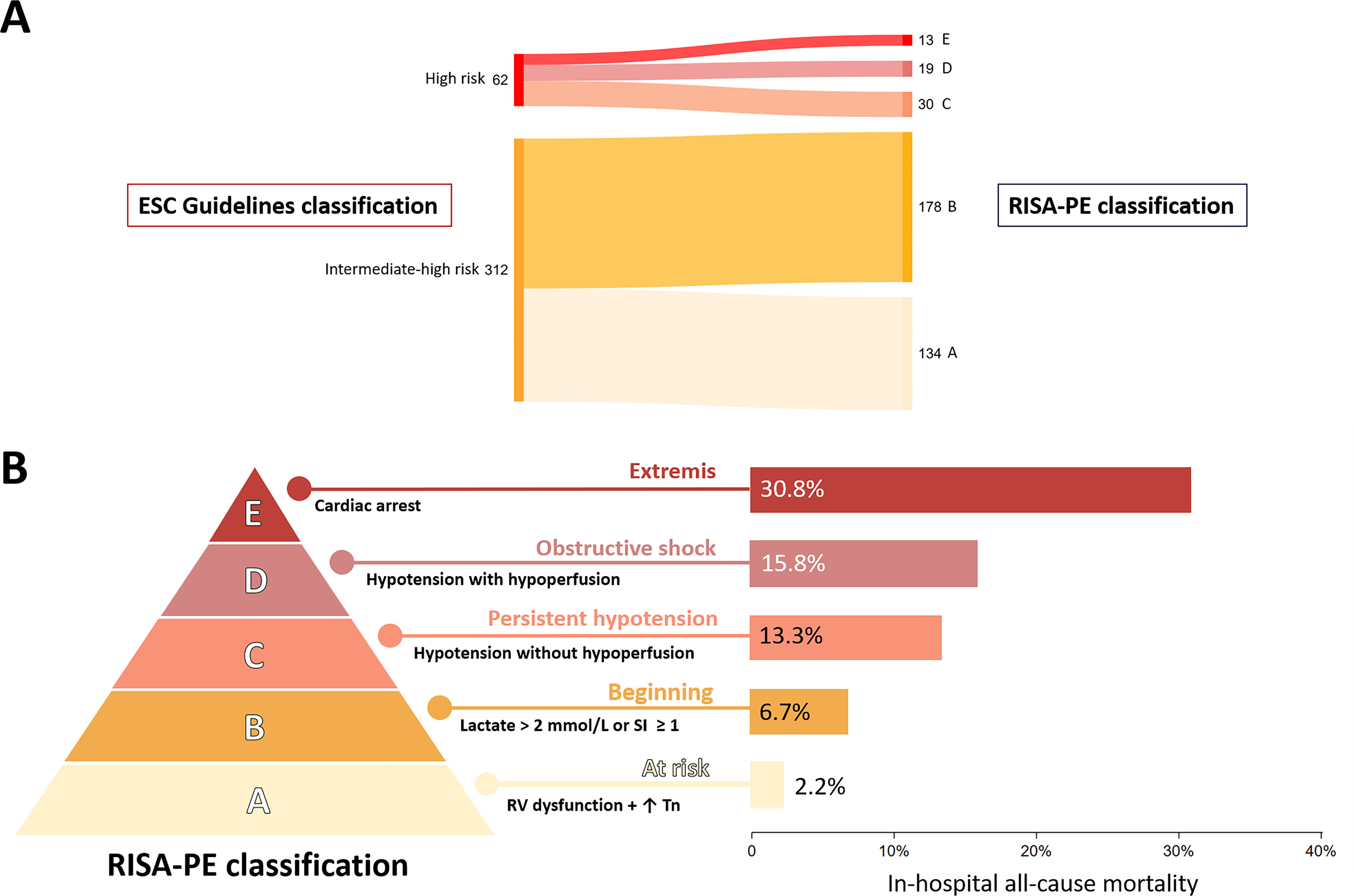
The study flow chart is presented in Supplementary Fig. 1. A total of 374 patients with IHR (n=312, 83.4%) or HR (n=62, 16.6%) PE were included in the final analysis (55.2% women; mean age, 64.2±17.6 years). The reclassification of patients from ESC risk stratification to RISA-PE stages is illustrated in Fig. 1A. Table 1 shows the baseline characteristics and clinical presentation according to RISA-PE stage. In stage E, post-resuscitation hemodynamic parameters were included. There were no between-stage significant differences in sex, age, or cardiovascular risk factors. However, PE severity parameters progressively increased with higher RISA-PE stages. Among the 62 patients stratified as HR according to ESC Guidelines, 24 (38.7%) received reperfusion therapy with ST.
RISA-PE classification: reclassification and in-hospital all-cause mortality. (A) Sankey diagram demonstrating the reclassification of patients with acute pulmonary embolism according to the ESC 2019 guidelines and the RISA-PE. (B) RISA-PE classification and in-hospital all-cause mortality. ESC, European Society of Cardiology; RISA-PE, RIsk claSsification Adapting SCAI shock stage to right ventricular failure in pulmonary embolism; RV, right ventricle; SI, shock index (heart rate/systolic blood pressure); Tn, troponin.
Baseline characteristics and clinical presentation at hospital admission according to RISA-PE stage.
| Total | A | B | C | D | E | p-Value | |
|---|---|---|---|---|---|---|---|
| N=374 | N=134 | N=178 | N=30 | N=19 | N=13 | ||
| Baseline characteristics | |||||||
| Male sex | 167 (44.8%) | 65 (48.5%) | 76 (42.9%) | 14 (46.7%) | 7 (36.8%) | 5 (38.5%) | 0.785 |
| Age, years | 64.2 (17.6) | 64.3 (16.7) | 64.0 (17.9) | 69.8 (17.7) | 60.3 (18.6) | 57.8 (20.0) | 0.230 |
| Body mass index, kg/m2 | 29.4 (7.1) | 29.9 (6.6) | 29.8 (8.1) | 26.2 (6.0) | 27.0 (5.2) | 27.8 (2.4) | 0.435 |
| Diabetes mellitus | 58 (18.5%) | 15 (13.5%) | 33 (23.2%) | 5 (17.2%) | 2 (10.5%) | 3 (23.1%) | 0.291 |
| Tobacco use | 96 (30.6%) | 35 (31.5%) | 43 (30.3%) | 7 (24.1%) | 7 (36.8%) | 4 (30.8%) | 0.914 |
| COPD | 41 (11.0%) | 12 (9.0%) | 22 (12.5%) | 4 (13.3%) | 1 (5.3%) | 2 (15.4%) | 0.730 |
| Prior VTE | 58 (15.6%) | 25 (18.8%) | 29 (16.4%) | 1 (3.4%) | 0 (0.0%) | 3 (23.1%) | 0.077 |
| Previous cancer | 56 (15.0%) | 16 (11.9%) | 28 (15.7%) | 7 (23.3%) | 3 (15.8%) | 2 (15.4%) | 0.608 |
| Clinical presentation at hospital admission | |||||||
| Syncope at presentation | 76 (24.3%) | 11 (9.9%) | 37 (26.1%) | 9 (32.1%) | 13 (68.4%) | 6 (46.2%) | <0.001 |
| Systolic blood pressure, mmHga | 120.2 (23.4) | 132.6 (17.5) | 116.4 (21.9) | 108.6 (23.4) | 88.6 (19.4) | 113.1 (28.3) | <0.001 |
| Vasoactive agents | 27 (7.2%) | – | – | 9 (31.0%) | 10 (52.6%) | 8 (66.7%) | <0.001 |
| Heart rate, beats/min | 106.8 (20.1) | 99.7 (17.2) | 111.4 (19.4) | 98.7 (21.4) | 118.0 (20.8) | 119.1 (24.5) | <0.001 |
| Shock index | 0.93 (0.30) | 0.76 (0.15) | 0.99 (0.26) | 0.96 (0.33) | 1.42 (0.49) | 1.11 (0.36) | <0.001 |
| Lactate, mmol/L | 3.0 (4.3) | 1.3 (0.4) | 3.3 (2.9) | 2.7 (2.6) | 8.3 (12.2) | 8.3 (3.8) | <0.001 |
| PESI score | 107.6 (33.9) | 95.4 (29.9) | 109.5 (32.7) | 126.0 (30.0) | 137.7 (35.2) | 123.7 (43.1) | <0.001 |
Data are shown as mean±SD for continuous variables and number (%) for categorical variables. p values denote the significance of the differences between the groups for continuous variables analyzed by the ANOVA test. The Chi-square Test tested the significance of between-group differences for categorical variables.
Overall, 26 patients (7.0%) died during hospitalization. In-hospital mortality increased progressively across RISA-PE stages: 2.2%, 6.7%, 13.3%, 15.8% and 30.8% for stages A–E, respectively, p-value for linear trend<0.001 (Fig. 1B). In patients treated only with anticoagulation (sensitivity analysis excluding patients who received ST), the differences in all-cause mortality across RISA-PE stages were more pronounced: 2.2%, 6.7%, 16.7%, 28.6%, and 57.1% for patients in stages A–E (p-value for linear trend<0.001). Using the ESC guidelines risk classification, the incidence of in-hospital all-cause mortality was 4.8% in patients with IHR-PE and 17.7% in patients with HR-PE (p-value<0.001). The RISA-PE showed a higher discrimination power as compared to ESC Guidelines risk classification (AUC 0.704 [95% confidence interval (CI): 0.606–0.803] vs. AUC 0.638 [95% CI: 0.540–0.737], respectively, p=0.022; difference: 0.066, 95% CI: 0.009–0.122) (Supplementary Fig. 2). The Brier score was lower for the RISA-PE (0.0612) compared to the ESC classification (0.0624), indicating a better calibration, with a non-statistically significant difference (p=0.380). Using the cut-off of A vs B-E in the RISA-PE classification, it demonstrated significantly higher sensitivity (88.5% vs 42.3%) and a lower (i.e. better) negative likelihood ratio (0.31 vs 0.68) compared to the ESC classification (IHR- vs HR-PE). Conversely, the ESC classification showed higher specificity and positive likelihood ratio. Compared with the ESC classification, RISA-PE showed a non-statistically significant reclassification improvement (IDI 0.018, p=0.210; NRI 0.225, p=0.268).
This study aimed to validate the RISA-PE classification in a multicenter cohort of patients with acute PE managed with medical therapy. Consistent with previous findings in patients treated with CDI [9], the RISA-PE classification demonstrated a stepwise increase in in-hospital all-cause mortality in this cohort of medically managed patients. Notably, the mortality gradient across the RISA-PE stages exhibited a more granular distribution compared to the binary ESC risk stratification, resulting in slightly improved discriminatory performance and modest, although non-significant, gains in calibration and reclassification. Notably, RISA-PE stage A was associated with a low in-hospital mortality rate (2.2%) under medical management, approximately half the mortality seen in the ESC-defined IHR group (4.8%). It is also worth noting that when comparing RISA-PE group A with the remaining strata (B–E), the sensitivity and negative predictive value for detecting in-hospital mortality were remarkably high. The RISA-PE relies on standard and easily obtainable clinical parameters, which may facilitate broader adoption in clinical practice. The development of new predictive risk classifications is crucial in this disease, as emerging therapies require accurate risk stratification to optimize patient outcomes.
The IHR-PE group includes patients with a wide spectrum of in-hospital mortality risk. In our cohort, this heterogeneity is evident, with 4.8% mortality in the IHR subgroup, consistent with real-world data reporting rates between 5% and 10% [8,10]. Mortality was 2.2% in stage A, resembling that of intermediate-low risk PE (1.7–2.1%) [11]. This finding suggests that anticoagulation with monitoring and possible rescue reperfusion may be appropriate in this subgroup. Conversely, mortality was 6.7% in stage B, yet data from registries and trials suggest this could be reduced to ∼1% with elective CDI [12,13]. This highlights a critical gap: IHR-PE patients with additional risk markers still face high mortality rates under conservative treatment, despite being traditionally perceived as stable patients. Known predictors of early PE-related death include tachycardia, hypotension, RV dysfunction, and elevated troponin or lactate [14–16]. Notably, a post hoc analysis of the FLASH registry linked some of these parameters to normotensive shock among patients with IHR-PE [6]. Both arterial and venous lactate elevation improves risk stratification in normotensive patients and is therefore included in RISA-PE [5,17]. The shock index is another independent predictor of 30-day mortality [18], reflecting pre-shock physiology [9]. These dynamic, easily measured variables are particularly valuable for inclusion in scoring tools. Ongoing trials such as PEERLESS II (NCT06055920) and HI-PEITHO (NCT04790370), will clarify whether CDI improves outcomes in IHR-PE patients with additional risk parameters. Positive results would support the clinical utility of the RISA-PE model as a stratification tool for identifying this higher-risk IHR subpopulation.
The presence of persistent hypotension (i.e., systolic blood pressure<90mmHg for ≥15min) defines HR-PE according to the ESC Guidelines [1]. However, there is also heterogeneity in this group, with Guidelines distinguishing three presentations: persistent hypotension, obstructive shock, and cardiac arrest. In a prospective registry, the in-hospital mortality rates for these presentations were 9.1%, 18.4%, and 59.5%, respectively [19], in line with our results. Additionally, as previously discussed, concern over bleeding complications often leads to the underuse of ST in HR-PE patients, which was also observed in our study (ST was administered to only one-third of HR-PE patients). This underscores the challenge of managing HR-PE patients. In the FLAME study, in-hospital mortality was 1.9% in patients treated with CDI vs. 29.5% in the comparator group, though baseline differences, such as higher cardiac arrest rates (stage E) in the control arm, limit direct comparison [20]. These findings might support HR-PE subclassification. RISA-PE stages C and some D may benefit from CDI if promptly transferred. In contrast, stage E poses major logistical challenges, often requiring mechanical support and/or immediate ST.
This study has several limitations. There is no strict time stamp for measurement of vital signs and biomarkers with respect to time of PE confirmation, although they were mostly measured at the time PE diagnosis in the emergency department. Lactate measurements were included irrespective of whether they were arterial or venous; therefore, potential differences between the two could not be evaluated. Due to its observational design, unmeasured confounders cannot be excluded. The total number of PE admissions was not recorded, introducing potential survival bias. Additionally, as a self-reported registry without external monitoring, data accuracy relied on local investigators.
This study adds validation to the RISA-PE classification in a cohort of medically managed patients with acute IHR and HR-PE. RISA-PE demonstrated a stepwise increase in in-hospital mortality across its stages, and appeared to provide higher discriminatory power compared to the ESC classification. It incorporates easily obtainable clinical parameters, supporting its feasibility for routine use in clinical practice. Notably, it identifies a low-risk subgroup (stage A) with mortality rates similar to intermediate-low risk PE, who may be treated conservatively. Also, it includes different subgroups of patients, both hemodynamically stable and unstable (i.e. stages B, C and D), which could potentially benefit from CDI to improve outcomes.
Author's contributionCR, RP, MEV-A, CR-L, RM, GG-M, BD-A, CF, AV-T, MJ, FJN, LJ-P, DJ and PS acquired the data. CR, RP, LJ-P, DJ and PS collaborated in the design, analysis and interpretation of data for the work. CR, RP, LJ-P, DJ and PS wrote the first draft of the manuscript. MEV-A, CR-L, RM, GG-M, BD-A, CF, AV-T, MJ and FJN critically reviewed the manuscript and approved the final version.
Artificial intelligence involvementAn artificial intelligence language model (ChatGPT) was used under human supervision to check the grammar and improve the readability of the English text. The authors take full responsibility for the content of the manuscript.
FundingNo specific funding was received for this study. The PROTECT study was funded by FIS 2008 (PI 08200), SEPAR 2008, NM 2010.
Conflicts of interestThe authors report no conflicts of interest with respect to the content of this manuscript.