Recent data from real world clinical practices on the use of Triple Therapy (TT) in patients with COPD are scarce.
MethodsObservational population-based study with longitudinal follow-up in patients with COPD identified in a primary care electronic medical records database in Catalonia, covering 80% of the general population. The aims were to characterize COPD patients who initiated TT and to describe treatment pathways before and after TT initiation. Time to and probability of step down or complete discontinuation of TT was described using restricted mean survival time and Kaplan–Meier analysis.
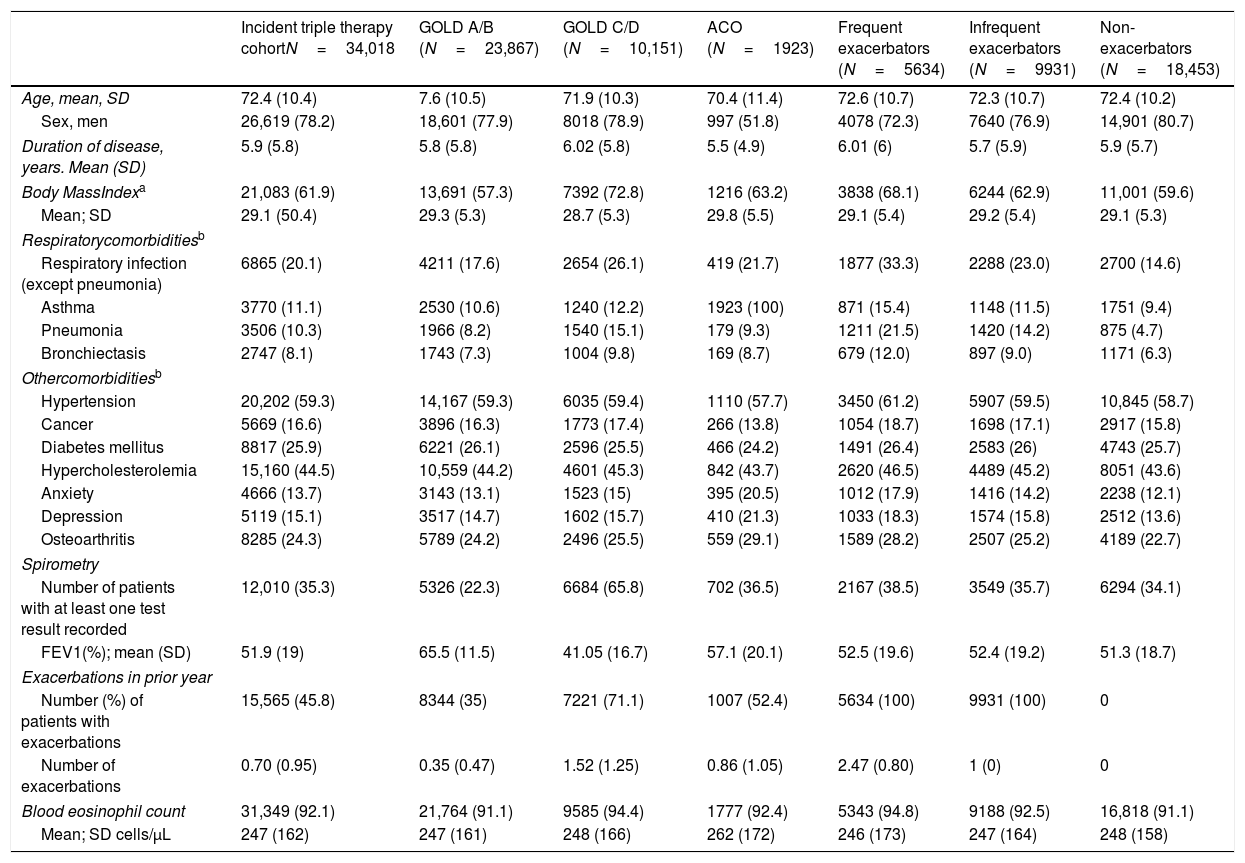
ResultsA total of 34,018 COPD patients initiated TT during the study period. Of them, 23,867 (70.1%) were GOLD A/B. 18,453 (54.2%) were non-exacerbators, 9931 (29.2%) infrequent exacerbators, 5634 (16.5%) frequent exacerbators and 1923 (5.6%) had asthma-COPD overlap. Drugs most frequently used prior to initiation of TT were long-acting antimuscarinics (22.5%) and combination of long-acting beta2 agonists/inhaled corticosteroids (15.2%). A total of 11,666 (34.3%) stepped down and 1091 (3.2%) discontinued TT during follow-up. Step down following TT was more likely in patients with severe COPD, especially during the first year; however, discontinuation was more common among patients with mild COPD.
ConclusionMost patients initiating treatment with TT were non exacerbators and continued on the same treatment over time regardless severity of disease. Stepping down was more frequent in severe patients, while discontinuation was more common among mild patients. Overall, it appears that TT is extensively used in primary care for treatment of patients with COPD.
Se dispone de pocos datos recientes de práctica clínica en el mundo real sobre el uso de la triple terapia (TT) en pacientes con EPOC.
MétodosEstudio observacional de base poblacional con seguimiento longitudinal en pacientes con EPOC identificados en una base de datos de historiales médicos electrónicos de atención primaria en Cataluña, que abarca el 80% de la población general. Los objetivos fueron caracterizar a los pacientes con EPOC que iniciaron la TT, y describir las vías de tratamiento antes y después del inicio de la TT. Se describió el tiempo y la probabilidad de desescalada o la suspensión completa de la TT utilizando el tiempo de supervivencia medio restringido y el análisis de Kaplan-Meier.
ResultadosUn total de 34.018 pacientes con EPOC iniciaron TT durante el período de estudio. De ellos, 23.867 (70,1%) eran GOLD A/B, 18.453 (54,2%) eran no exacerbadores, 9.931 (29,2%) exacerbadores infrecuentes, 5.634 (16,5%) exacerbadores frecuentes y 1.923 (5,6%) presentaban superposición asma-EPOC. Los fármacos que se usaron con mayor frecuencia antes del inicio de la TT fueron los antimuscarínicos de acción prolongada (22,5%), y la combinación de agonistas beta2 de acción prolongada/corticosteroides inhalados (15,2%). Un total de 11.666 pacientes (34,3%) desescalaron la TT y 1.091 (3,2%) suspendieron el tratamiento durante el seguimiento. La desescalada después de la TT fue más probable en pacientes con EPOC grave, especialmente durante el primer año; sin embargo, la suspensión fue más común en pacientes con EPOC leve.
ConclusiónLa mayoría de los pacientes que iniciaron el tratamiento con la TT eran no exacerbadores y continuaron con el mismo tratamiento, independientemente de la gravedad de la enfermedad. La desescalada fue más frecuente en pacientes graves, mientras que la suspensión fue más común entre los pacientes leves. En general, parece que la TT se utiliza ampliamente en el nivel de atención primaria para el tratamiento de pacientes con EPOC.
Chronic Obstructive Pulmonary Disease (COPD) is characterized by persistent airflow limitation and is a major cause of mortality and morbidity worldwide.1 The aim of treatment of COPD is to reduce symptoms, decrease the frequency and severity of exacerbations and improve exercise tolerance and quality of life.2,3 Pharmacological management is based on long-acting bronchodilators (LABD), either a long-acting muscarinic antagonist (LAMA) or a long-acting β2-agonist (LABA) or both, and inhaled corticosteroids (ICS).2,3 Current recommendations suggest to start with one LABD and step up to dual or triple therapy (TT) (ICS/LABA/LAMA), as necessary.2 Therefore; TT is recommended only for patients with frequent exacerbations whose symptoms are not adequately controlled with either LABA/LAMA or ICS/LABA combination.2,4
Despite these recommendations, TT is used extensively in current clinical practice, as has been reported in several studies around the world.5–9 Since TT is becoming increasingly important in clinical practice and over-prescription of inhaled medication is associated with increased costs and possible risk of side effects,10 it's important to understand the current situation of COPD patients with TT in real life and to evaluate the treatment pathways leading to TT. While some studies have been conducted to understand patient characteristics and factors related to TT use,7,11,12 and the efficacy and tolerability of TT have been evaluated in several clinical trials,13–15 there is limited “real world” information regarding pathways before and after initiation of TT.
The objectives of this study were to characterize COPD patients who initiated TT and to describe treatment pathways before and after TT initiation in a population-based sample of patients with COPD in Primary Care.
MethodsStudy designThis was a retrospective study with longitudinal follow-up using patient-level primary care data from 2011 to 2015. The data were obtained from the Information System for the Development of Research in Primary Care (SIDIAP) database, which contains anonymized computerized primary care medical records from 5.8 million people in Catalonia (Spain), which represents more than 80% of the total population.16 This database has been used and validated for epidemiological research in respiratory diseases.8,17
Patient selectionWe selected individuals older than 40 years, with at least one diagnosis of COPD (International Classification of Disease – 10th Edition [ICD-10] codes in Appendix A) and evidence of initiation of TT between January 1st, 2011 and December 31st, 2015. Initiation of TT was defined as having prescriptions for LAMA, LABA, and ICS with an overlap for ≥30 days. The date of prescription of the third component was defined as the start of TT or index date and this was the start of the data collection period for each patient. All patients were required to have at least 12-months of available medical record data prior to this index date (“pre-index”). Patients with any evidence of TT prescriptions during the pre-index period were excluded from study.
Patient subgroupsPatients were classified according to the Global Initiative for Chronic Obstructive lung Disease (GOLD) 2014 categories based on spirometry and exacerbation data as low risk (GOLD A/B) and high risk (GOLD C/D).2
Patients were also classified according to clinical phenotypes.3 Those with two or more exacerbations during the year before initiation of triple therapy were classified as having a frequent exacerbator phenotype, patients with only one exacerbation were classified as infrequent exacerbators and the remaining COPD patients were considered non-exacerbators. Patients with a concomitant diagnosis of asthma were included in the asthma COPD overlap (ACO) phenotype.18,19
Patients with available forced expiratory volume in one second (FEV1) results were classified into different levels of airflow limitation according to the most proximal FEV1 test results around index date (stage 1 mild, FEV≥80% predicted, stage 2 moderate, 50%≤FEV1<80% predicted; stage 3 severe, 30%≤FEV1<50% predicted; stage 4 very severe, FEV<30% predicted).
Study measurementsThe following variables were obtained at baseline: demographic and clinical characteristics, including, age, sex, smoking history, comorbidities and episodes of pneumonia previous to the index date. Diagnostic spirometry, exacerbations, healthcare utilization and treatments received during the year before inclusion in the study were also collected. The blood analysis closest to the index date was selected to obtain the blood eosinophils values.
Exacerbation episodes were identified using outpatient visits with a diagnostic code indicative of a respiratory exacerbation, or receipt of corticosteroids and/or antibiotics used for treating exacerbations, in accordance with previously published algorithms.8,17 Exacerbations leading to hospitalizations or treated as part of inpatient care were not available in the data. To avoid misclassification and over-estimation of exacerbations, consecutive episodes with less than 21 days between prescriptions or general practitioner (GP) visits were considered as a single event.
Patients will be considered as having a step-down if they have discontinued on at least one of the three compounds LABA, LAMA, or ICS, defined as having no subsequent prescriptions during the 60 days from initiation of triple therapy, AND they had a subsequent prescription for at least one of the three compounds that is different than the one that was discontinued.
A 60-day “gap” in subsequent prescriptions for all three components was considered as a discontinuation from TT. Patients who neither discontinue nor stepped down during follow-up were considered persistent on TT.
The study was approved by the Research and Ethics Committee of the IDIAP Jordi Gol Institute of Research In Primary Care (Barcelona, Spain). Since anonymised data were collected retrospectively, no informed consent was considered necessary.
Statistical analysisA descriptive analysis was performed with baseline sociodemographic, clinical characteristics and baseline treatments of patients included. Categorical variables were described using absolute frequencies and corresponding percentages. Continuous variables following a normal distribution were described using the mean and standard deviation (SD), while those that did not follow a normal distribution were describing using the median and interquartile range. Pathways and treatment following TT were presented as absolute frequencies and percentages.
Time to and probability of first event (step down or complete discontinuation) was described using restricted mean survival time (the average time-to-event in a restricted time-period) and using Kaplan–Meier analysis over 5 years of follow-up. The same analyses were performed according to GOLD severity and phenotype. All statistical analyses were performed using the statistical software package (SPSS version 20.0, IBM, Chicago, IL, USA).
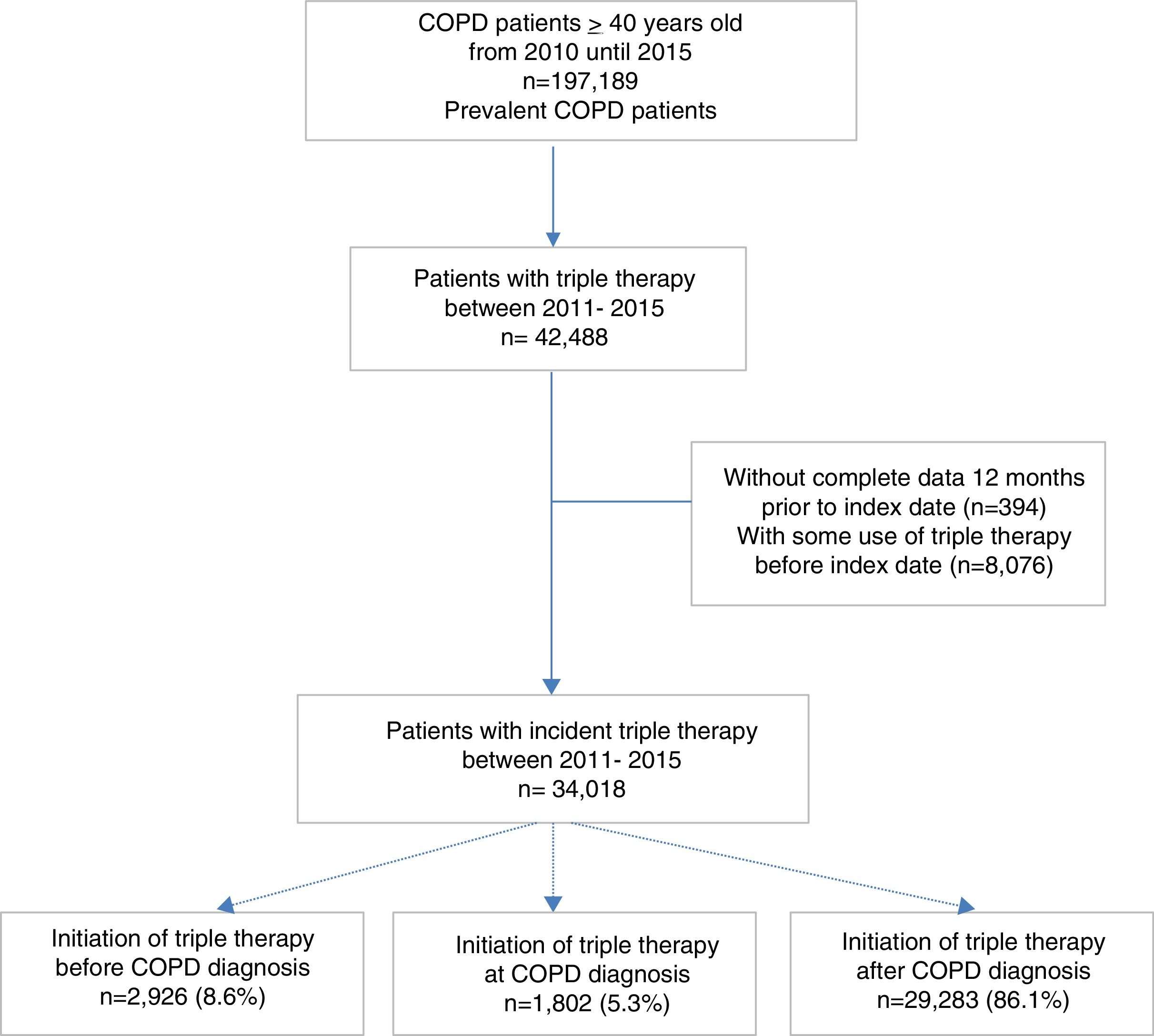
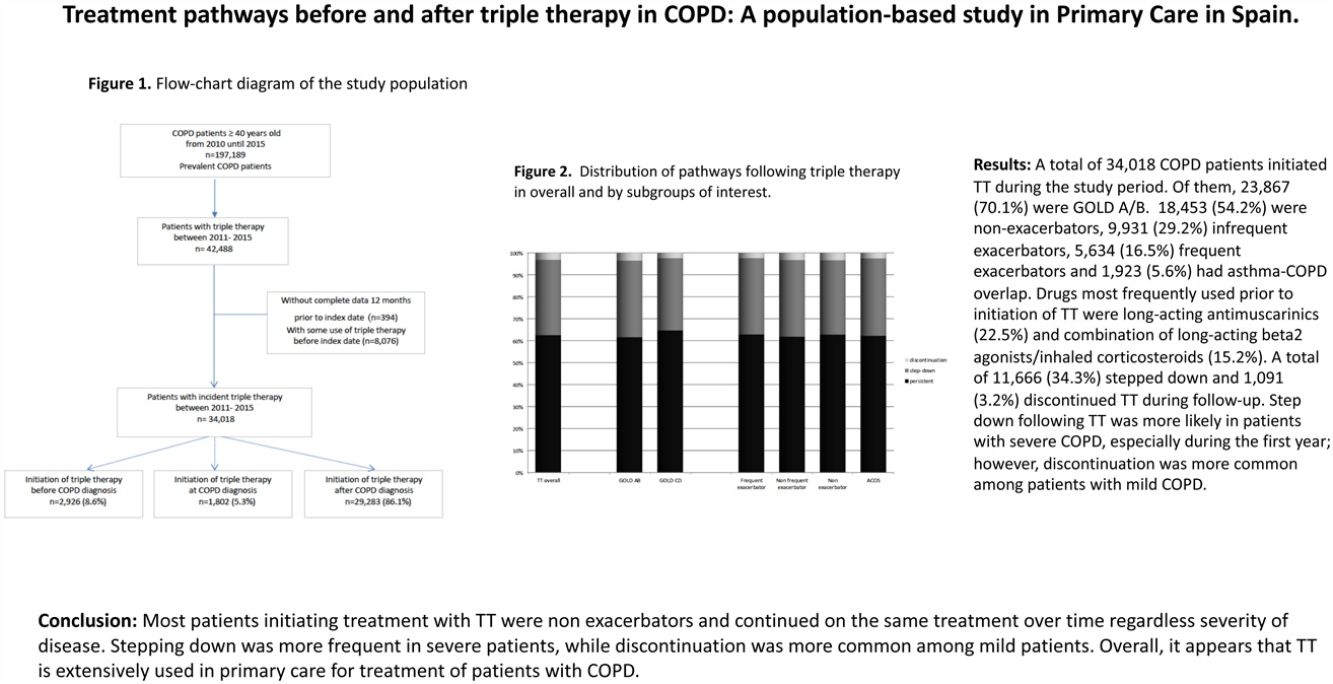
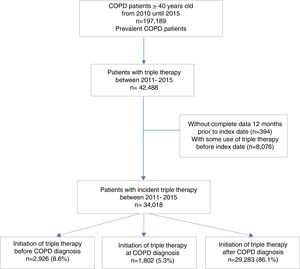
ResultsDuring the study period, a total of 197,189 patients with recorded diagnosis of COPD were identified; of whom 34,018 (17.2%) initiated TT and constituted the study population. Among these patients, 8.6% initiated TT prior to COPD diagnosis, 5.3% at COPD diagnosis and 86.1% after COPD diagnosis (Fig. 1).
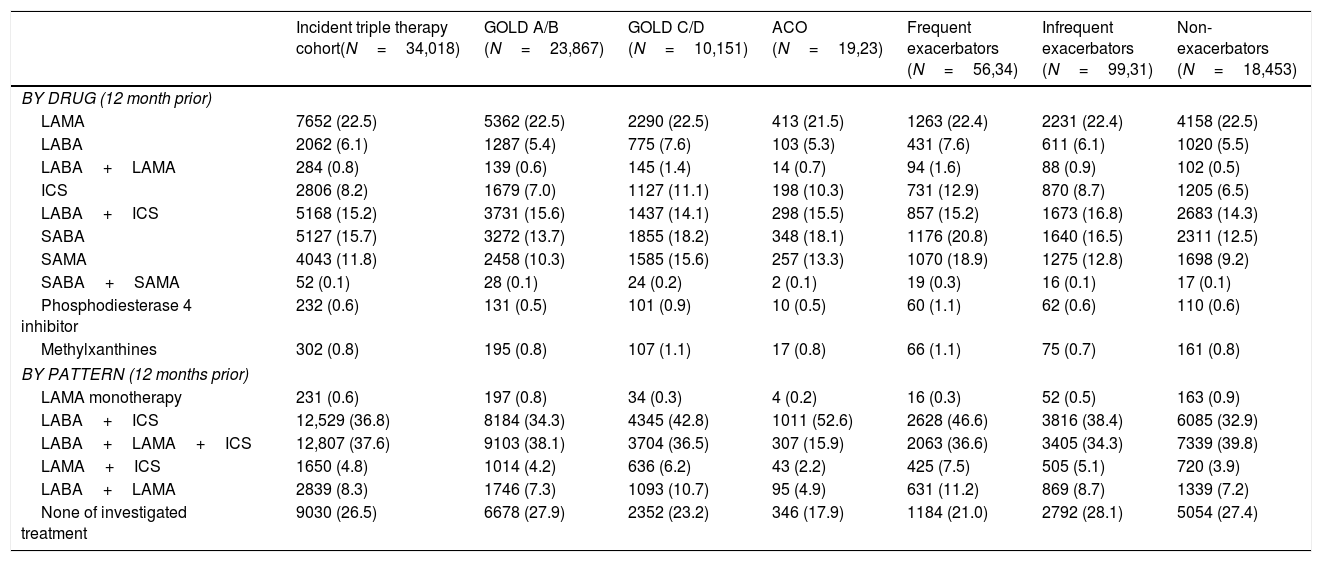
Baseline characteristics at initiation of triple therapyIn total, 26,619 (78.2%) patients were men. Mean age was 72.4 years (SD: 10.4) and mean duration of disease was 5.9 years (SD: 5.8). Up to 92% of patients had blood analysis performed during the year before TT initiation and mean eosinophil count was 247.6cells/μl (SD: 162.6); in contrast, only 35.3% of patients had spirometry and mean FEV1 (% predicted) was 51.9% (SD: 19%).
The majority of patients who initiated TT (N=23,867, 70.1%) were GOLD A/B. Regarding phenotype, most patients were non-exacerbators 18,453 (54.2%) followed by infrequent exacerbators 9931 (29.2%) and the remaining 5634 patients (16.5%) were frequent exacerbators. Of all patients, 1923 (5.6%) were classified as having ACO (Table 1).
Baseline Characteristics of incident triple therapy cohort (12 months prior and at index date), for all patients and by subgroup of interest.
| Incident triple therapy cohortN=34,018 | GOLD A/B (N=23,867) | GOLD C/D (N=10,151) | ACO (N=1923) | Frequent exacerbators (N=5634) | Infrequent exacerbators (N=9931) | Non-exacerbators (N=18,453) | |
|---|---|---|---|---|---|---|---|
| Age, mean, SD | 72.4 (10.4) | 7.6 (10.5) | 71.9 (10.3) | 70.4 (11.4) | 72.6 (10.7) | 72.3 (10.7) | 72.4 (10.2) |
| Sex, men | 26,619 (78.2) | 18,601 (77.9) | 8018 (78.9) | 997 (51.8) | 4078 (72.3) | 7640 (76.9) | 14,901 (80.7) |
| Duration of disease, years. Mean (SD) | 5.9 (5.8) | 5.8 (5.8) | 6.02 (5.8) | 5.5 (4.9) | 6.01 (6) | 5.7 (5.9) | 5.9 (5.7) |
| Body MassIndexa | 21,083 (61.9) | 13,691 (57.3) | 7392 (72.8) | 1216 (63.2) | 3838 (68.1) | 6244 (62.9) | 11,001 (59.6) |
| Mean; SD | 29.1 (50.4) | 29.3 (5.3) | 28.7 (5.3) | 29.8 (5.5) | 29.1 (5.4) | 29.2 (5.4) | 29.1 (5.3) |
| Respiratorycomorbiditiesb | |||||||
| Respiratory infection (except pneumonia) | 6865 (20.1) | 4211 (17.6) | 2654 (26.1) | 419 (21.7) | 1877 (33.3) | 2288 (23.0) | 2700 (14.6) |
| Asthma | 3770 (11.1) | 2530 (10.6) | 1240 (12.2) | 1923 (100) | 871 (15.4) | 1148 (11.5) | 1751 (9.4) |
| Pneumonia | 3506 (10.3) | 1966 (8.2) | 1540 (15.1) | 179 (9.3) | 1211 (21.5) | 1420 (14.2) | 875 (4.7) |
| Bronchiectasis | 2747 (8.1) | 1743 (7.3) | 1004 (9.8) | 169 (8.7) | 679 (12.0) | 897 (9.0) | 1171 (6.3) |
| Othercomorbiditiesb | |||||||
| Hypertension | 20,202 (59.3) | 14,167 (59.3) | 6035 (59.4) | 1110 (57.7) | 3450 (61.2) | 5907 (59.5) | 10,845 (58.7) |
| Cancer | 5669 (16.6) | 3896 (16.3) | 1773 (17.4) | 266 (13.8) | 1054 (18.7) | 1698 (17.1) | 2917 (15.8) |
| Diabetes mellitus | 8817 (25.9) | 6221 (26.1) | 2596 (25.5) | 466 (24.2) | 1491 (26.4) | 2583 (26) | 4743 (25.7) |
| Hypercholesterolemia | 15,160 (44.5) | 10,559 (44.2) | 4601 (45.3) | 842 (43.7) | 2620 (46.5) | 4489 (45.2) | 8051 (43.6) |
| Anxiety | 4666 (13.7) | 3143 (13.1) | 1523 (15) | 395 (20.5) | 1012 (17.9) | 1416 (14.2) | 2238 (12.1) |
| Depression | 5119 (15.1) | 3517 (14.7) | 1602 (15.7) | 410 (21.3) | 1033 (18.3) | 1574 (15.8) | 2512 (13.6) |
| Osteoarthritis | 8285 (24.3) | 5789 (24.2) | 2496 (25.5) | 559 (29.1) | 1589 (28.2) | 2507 (25.2) | 4189 (22.7) |
| Spirometry | |||||||
| Number of patients with at least one test result recorded | 12,010 (35.3) | 5326 (22.3) | 6684 (65.8) | 702 (36.5) | 2167 (38.5) | 3549 (35.7) | 6294 (34.1) |
| FEV1(%); mean (SD) | 51.9 (19) | 65.5 (11.5) | 41.05 (16.7) | 57.1 (20.1) | 52.5 (19.6) | 52.4 (19.2) | 51.3 (18.7) |
| Exacerbations in prior year | |||||||
| Number (%) of patients with exacerbations | 15,565 (45.8) | 8344 (35) | 7221 (71.1) | 1007 (52.4) | 5634 (100) | 9931 (100) | 0 |
| Number of exacerbations | 0.70 (0.95) | 0.35 (0.47) | 1.52 (1.25) | 0.86 (1.05) | 2.47 (0.80) | 1 (0) | 0 |
| Blood eosinophil count | 31,349 (92.1) | 21,764 (91.1) | 9585 (94.4) | 1777 (92.4) | 5343 (94.8) | 9188 (92.5) | 16,818 (91.1) |
| Mean; SD cells/μL | 247 (162) | 247 (161) | 248 (166) | 262 (172) | 246 (173) | 247 (164) | 248 (158) |
The data are n (%), unless otherwise indicated. SD, standard deviation; ACO, asthma COPD overlap; FEV1, forced expiratory volume in 1s; GOLD, global initiative for obstructive chronic pulmonary disease.
The most frequent respiratory-related comorbidities were respiratory infections (20.2%), pneumonia (10.3%) and bronchiectasis (8.1%). The most frequent non-respiratory comorbidities were hypertension (59.4%), hypercholesterolemia (44.6%), obesity (26.6%) and diabetes mellitus (25.5%). The main characteristics of the population are described in detail in Table 1.
Treatment used before triple therapyDrugs more frequently used prior to initiation of TT were LAMA, LABA/ICS and ICS (22.5%, 15.2% and 8.2%, respectively).
Use of LABA/ICS before TT was most frequent among patients with ACO (44.3%), frequent exacerbators (36.9%) and GOLD C/D (34.3%) patients; while the use of LABA+LAMA+ICS at any time in the previous year, but overlapping less than 30 days (not fulfilling the definition of TT) was most frequent in frequent exacerbators (17.9%) and GOLD C/D (16.4%) patients. LABA/ICS was prescribed to 31.6% of infrequent exacerbators and 27.4% of non-exacerbators, whilst LABA+LAMA+ICS were prescribed to 14.1%, and 11.7%, respectively. (Table 2).
Treatment of incident triple therapy cohort during the 12 months prior to the index date. For all patients and by subgroup of interest.
| Incident triple therapy cohort(N=34,018) | GOLD A/B (N=23,867) | GOLD C/D (N=10,151) | ACO (N=19,23) | Frequent exacerbators (N=56,34) | Infrequent exacerbators (N=99,31) | Non-exacerbators (N=18,453) | |
|---|---|---|---|---|---|---|---|
| BY DRUG (12 month prior) | |||||||
| LAMA | 7652 (22.5) | 5362 (22.5) | 2290 (22.5) | 413 (21.5) | 1263 (22.4) | 2231 (22.4) | 4158 (22.5) |
| LABA | 2062 (6.1) | 1287 (5.4) | 775 (7.6) | 103 (5.3) | 431 (7.6) | 611 (6.1) | 1020 (5.5) |
| LABA+LAMA | 284 (0.8) | 139 (0.6) | 145 (1.4) | 14 (0.7) | 94 (1.6) | 88 (0.9) | 102 (0.5) |
| ICS | 2806 (8.2) | 1679 (7.0) | 1127 (11.1) | 198 (10.3) | 731 (12.9) | 870 (8.7) | 1205 (6.5) |
| LABA+ICS | 5168 (15.2) | 3731 (15.6) | 1437 (14.1) | 298 (15.5) | 857 (15.2) | 1673 (16.8) | 2683 (14.3) |
| SABA | 5127 (15.7) | 3272 (13.7) | 1855 (18.2) | 348 (18.1) | 1176 (20.8) | 1640 (16.5) | 2311 (12.5) |
| SAMA | 4043 (11.8) | 2458 (10.3) | 1585 (15.6) | 257 (13.3) | 1070 (18.9) | 1275 (12.8) | 1698 (9.2) |
| SABA+SAMA | 52 (0.1) | 28 (0.1) | 24 (0.2) | 2 (0.1) | 19 (0.3) | 16 (0.1) | 17 (0.1) |
| Phosphodiesterase 4 inhibitor | 232 (0.6) | 131 (0.5) | 101 (0.9) | 10 (0.5) | 60 (1.1) | 62 (0.6) | 110 (0.6) |
| Methylxanthines | 302 (0.8) | 195 (0.8) | 107 (1.1) | 17 (0.8) | 66 (1.1) | 75 (0.7) | 161 (0.8) |
| BY PATTERN (12 months prior) | |||||||
| LAMA monotherapy | 231 (0.6) | 197 (0.8) | 34 (0.3) | 4 (0.2) | 16 (0.3) | 52 (0.5) | 163 (0.9) |
| LABA+ICS | 12,529 (36.8) | 8184 (34.3) | 4345 (42.8) | 1011 (52.6) | 2628 (46.6) | 3816 (38.4) | 6085 (32.9) |
| LABA+LAMA+ICS | 12,807 (37.6) | 9103 (38.1) | 3704 (36.5) | 307 (15.9) | 2063 (36.6) | 3405 (34.3) | 7339 (39.8) |
| LAMA+ICS | 1650 (4.8) | 1014 (4.2) | 636 (6.2) | 43 (2.2) | 425 (7.5) | 505 (5.1) | 720 (3.9) |
| LABA+LAMA | 2839 (8.3) | 1746 (7.3) | 1093 (10.7) | 95 (4.9) | 631 (11.2) | 869 (8.7) | 1339 (7.2) |
| None of investigated treatment | 9030 (26.5) | 6678 (27.9) | 2352 (23.2) | 346 (17.9) | 1184 (21.0) | 2792 (28.1) | 5054 (27.4) |
The data are n (%), unless otherwise indicated. ICS, inhaled corticosteroid; LABA, long-acting b2 agonist; LAMA, long-acting antimuscarinic agent; SABA, short-acting b2-agonist; SAMA, short-acting muscarinic agent.
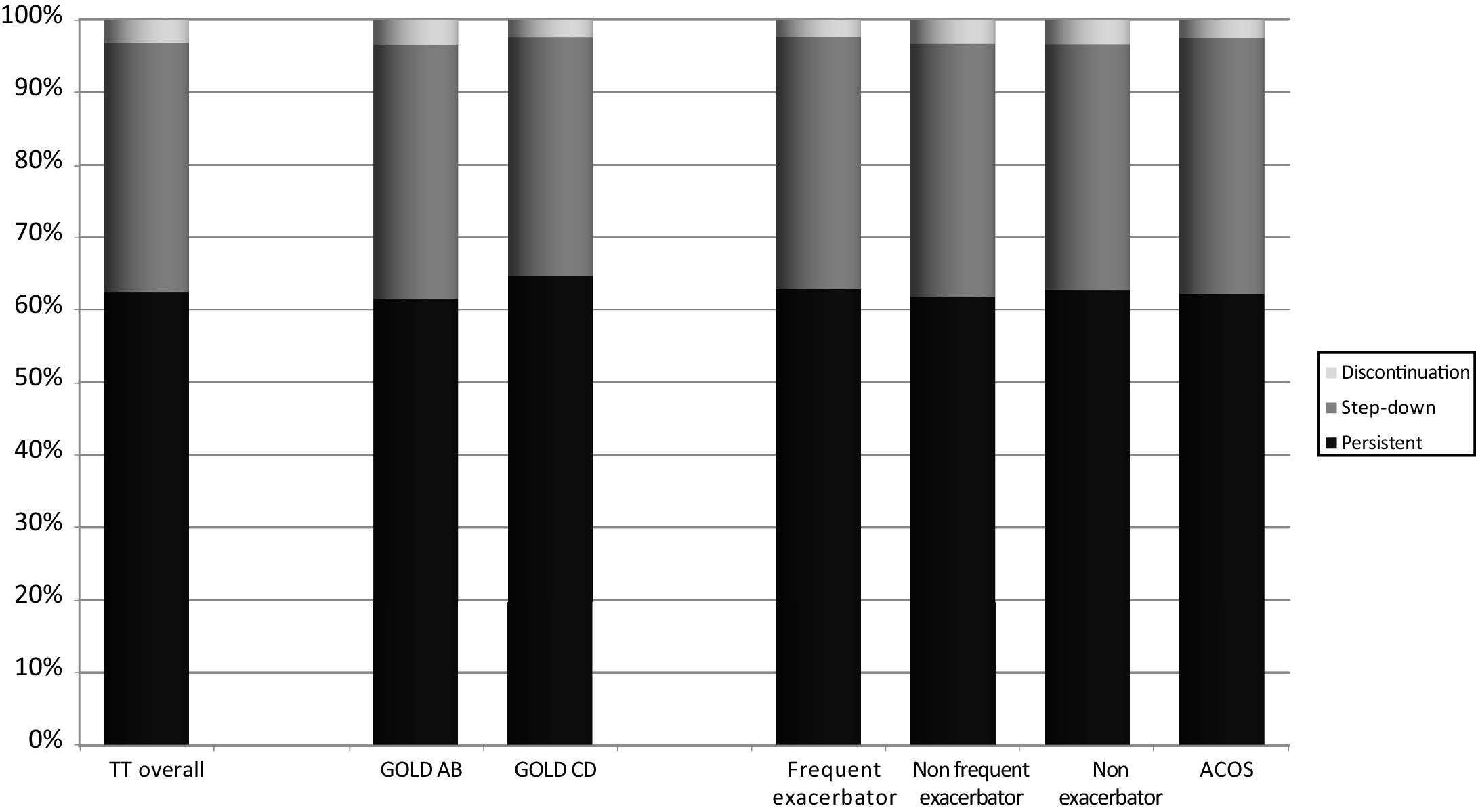
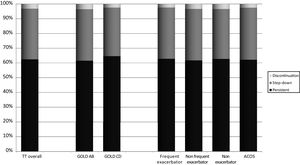
A total of 21,261 (62.5%) patients continued receiving TT throughout follow-up. However, 11,666 patients (34.3%) stepped down from TT and 1091 patients (3.2%) discontinued TT at any time during follow-up. Distribution of pathways following TT initiation was similar between subgroups (Fig. 2).
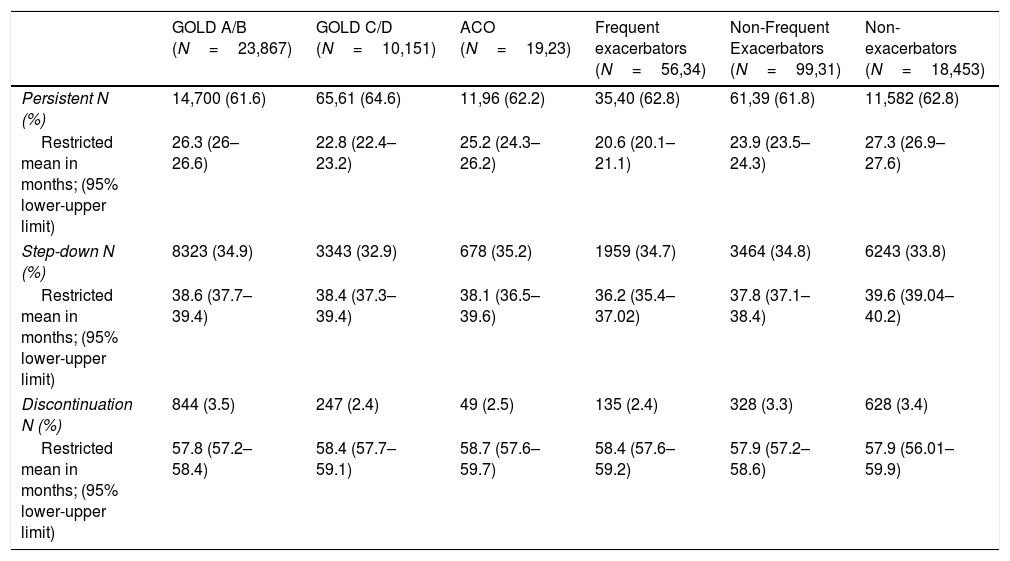
Time to step down or discontinuationOver 5 years of follow-up, 11,666 (34.3%) patients stepped down from TT. Of them, 7710 (66.1%) did so within one year of TT initiation, and 9984 (85.6%) and 11,059 (94.8%) after two and three years, respectively. Patterns of step down were similar across subgroups (data not shown). The restricted mean time to step down following TT initiation was 38.9 months (95%CI, 51.3–57.9) and was similar across GOLD subgroups. However, among phenotypes, frequent exacerbators stepped down around 3 months earlier (36.2 months) than non-exacerbators (39.6 months) (Table 3). On the other hand, 1091 (3.2%) patients presented complete discontinuation with a restricted mean time to discontinuation of 58.4 months (95%CI, NA), similar across subgroups (Table 3).
Time to first event following Triple Therapy in the Incident Triple Therapy Cohort, Overall and by Subgroup of Interest.
| GOLD A/B (N=23,867) | GOLD C/D (N=10,151) | ACO (N=19,23) | Frequent exacerbators (N=56,34) | Non-Frequent Exacerbators (N=99,31) | Non-exacerbators (N=18,453) | |
|---|---|---|---|---|---|---|
| Persistent N (%) | 14,700 (61.6) | 65,61 (64.6) | 11,96 (62.2) | 35,40 (62.8) | 61,39 (61.8) | 11,582 (62.8) |
| Restricted mean in months; (95% lower-upper limit) | 26.3 (26–26.6) | 22.8 (22.4–23.2) | 25.2 (24.3–26.2) | 20.6 (20.1–21.1) | 23.9 (23.5–24.3) | 27.3 (26.9–27.6) |
| Step-down N (%) | 8323 (34.9) | 3343 (32.9) | 678 (35.2) | 1959 (34.7) | 3464 (34.8) | 6243 (33.8) |
| Restricted mean in months; (95% lower-upper limit) | 38.6 (37.7–39.4) | 38.4 (37.3–39.4) | 38.1 (36.5– 39.6) | 36.2 (35.4–37.02) | 37.8 (37.1–38.4) | 39.6 (39.04–40.2) |
| Discontinuation N (%) | 844 (3.5) | 247 (2.4) | 49 (2.5) | 135 (2.4) | 328 (3.3) | 628 (3.4) |
| Restricted mean in months; (95% lower-upper limit) | 57.8 (57.2–58.4) | 58.4 (57.7–59.1) | 58.7 (57.6–59.7) | 58.4 (57.6–59.2) | 57.9 (57.2–58.6) | 57.9 (56.01–59.9) |
ACO, asthma COPD overlap; FEV1, forced expiratory volume in 1s; GOLD, global initiative for obstructive chronic pulmonary disease.
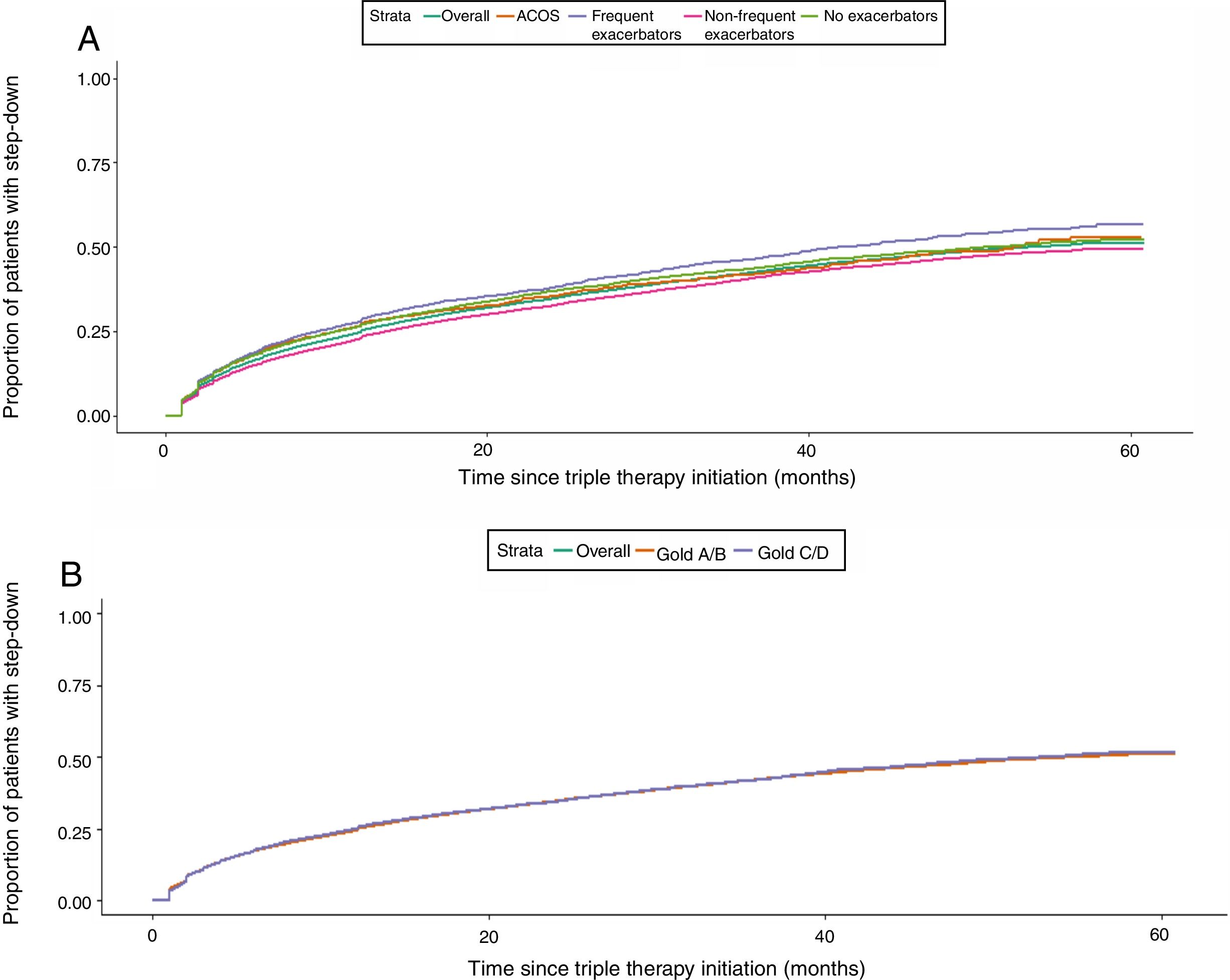
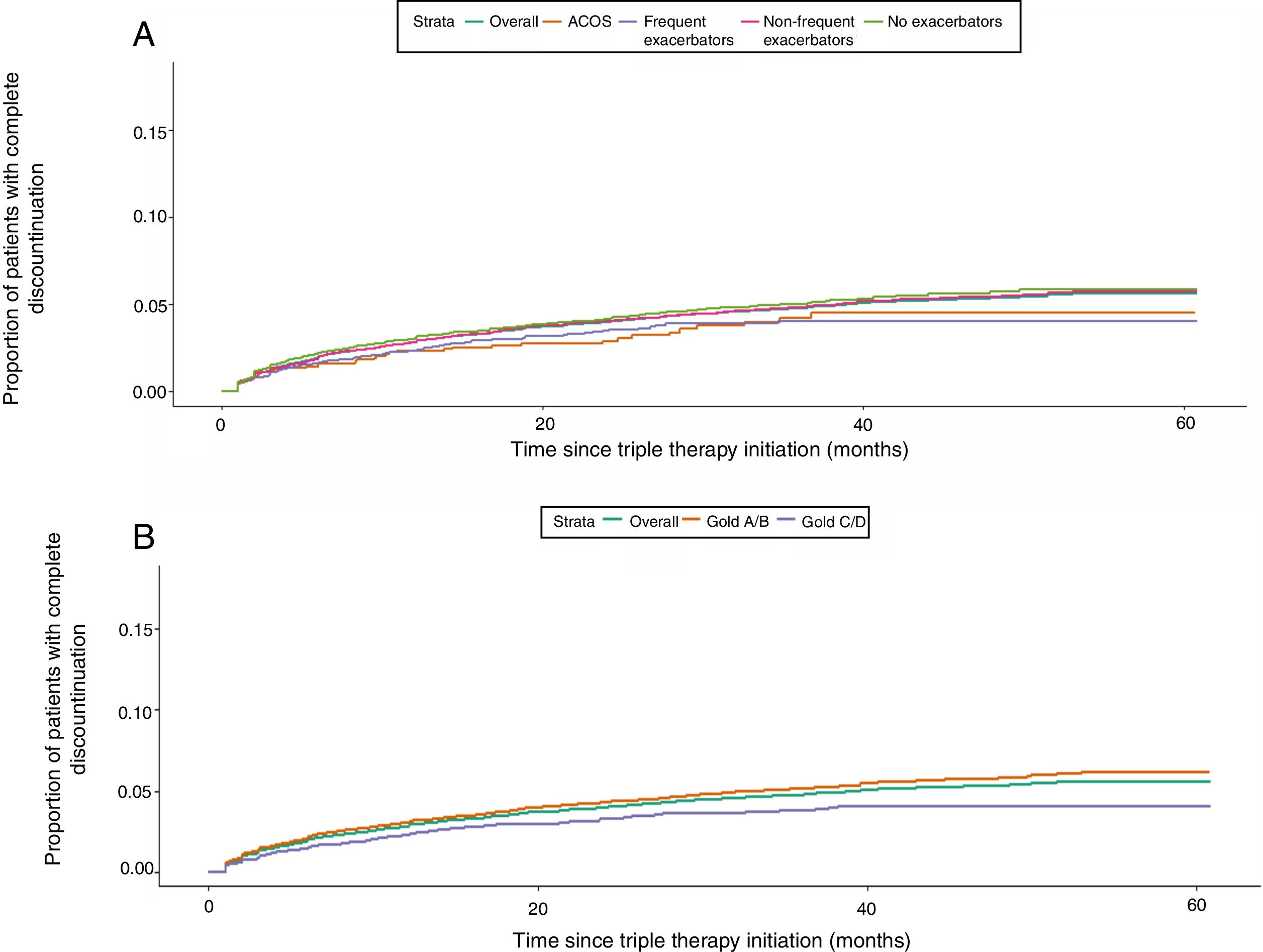
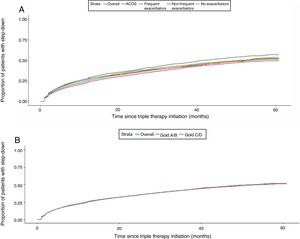
Kaplan Meier analysis showed that patients starting TT had a 50% probability of step down at 5 years of follow-up. Higher proportions of step downs were observed in frequent exacerbators and patients with ACO; however, observed proportions were similar across GOLD subgroups (Fig. 3). Probability of TT discontinuation within 5 years was about 6%, with higher proportion in milder patients (GOLD A/B, non-exacerbators and non-frequent exacerbators) (Fig. 4).
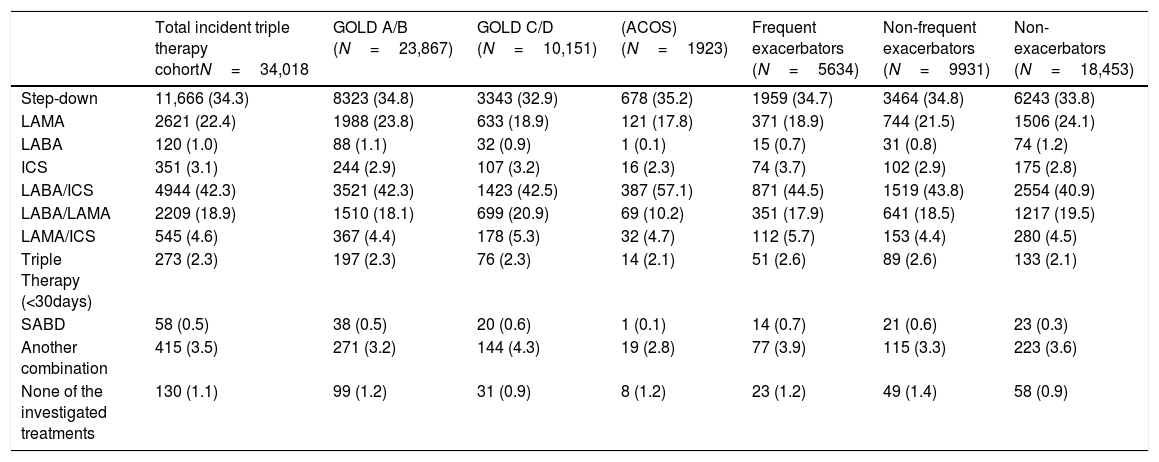
The COPD medications more frequently prescribed during the next 30 days after stepping down from TT were LABA/ICS (42.4%), LAMA (22.4%) and LABA/LAMA (18.9%). No significant differences were seen between subgroups (Table 4).
Treatment following triple therapy, Overall and by Subgroup of Interest.
| Total incident triple therapy cohortN=34,018 | GOLD A/B (N=23,867) | GOLD C/D (N=10,151) | (ACOS) (N=1923) | Frequent exacerbators (N=5634) | Non-frequent exacerbators (N=9931) | Non-exacerbators (N=18,453) | |
|---|---|---|---|---|---|---|---|
| Step-down | 11,666 (34.3) | 8323 (34.8) | 3343 (32.9) | 678 (35.2) | 1959 (34.7) | 3464 (34.8) | 6243 (33.8) |
| LAMA | 2621 (22.4) | 1988 (23.8) | 633 (18.9) | 121 (17.8) | 371 (18.9) | 744 (21.5) | 1506 (24.1) |
| LABA | 120 (1.0) | 88 (1.1) | 32 (0.9) | 1 (0.1) | 15 (0.7) | 31 (0.8) | 74 (1.2) |
| ICS | 351 (3.1) | 244 (2.9) | 107 (3.2) | 16 (2.3) | 74 (3.7) | 102 (2.9) | 175 (2.8) |
| LABA/ICS | 4944 (42.3) | 3521 (42.3) | 1423 (42.5) | 387 (57.1) | 871 (44.5) | 1519 (43.8) | 2554 (40.9) |
| LABA/LAMA | 2209 (18.9) | 1510 (18.1) | 699 (20.9) | 69 (10.2) | 351 (17.9) | 641 (18.5) | 1217 (19.5) |
| LAMA/ICS | 545 (4.6) | 367 (4.4) | 178 (5.3) | 32 (4.7) | 112 (5.7) | 153 (4.4) | 280 (4.5) |
| Triple Therapy (<30days) | 273 (2.3) | 197 (2.3) | 76 (2.3) | 14 (2.1) | 51 (2.6) | 89 (2.6) | 133 (2.1) |
| SABD | 58 (0.5) | 38 (0.5) | 20 (0.6) | 1 (0.1) | 14 (0.7) | 21 (0.6) | 23 (0.3) |
| Another combination | 415 (3.5) | 271 (3.2) | 144 (4.3) | 19 (2.8) | 77 (3.9) | 115 (3.3) | 223 (3.6) |
| None of the investigated treatments | 130 (1.1) | 99 (1.2) | 31 (0.9) | 8 (1.2) | 23 (1.2) | 49 (1.4) | 58 (0.9) |
The data are n (%), unless otherwise indicated. ICS, inhaled corticosteroid; LABA, long-acting b2 agonist; LAMA, long-acting muscarinic antagonists; SABA, short-acting b2-agonists; SAMA, short-acting muscarinic antagonists. SABD, Short acting bronchodilators.
The current study provides a description of the clinical profile of patients with COPD who initiated treatment with TT and treatment pathways prior and following TT initiation in real-life clinical practice. We observed that patients who initiate TT often receive ICS containing regimens as prior treatment and without having experienced exacerbations in the year prior, suggesting that less severe patients are receiving TT, in contrast to treatment recommendations. Treatment with TT tends to remain persistent irrespective of the severity of the disease and the level of risk. Step down following TT is more likely in severe patients, especially during the first year.
Prescription of TT for COPD patients is common in current clinical practice7,20–22 and it's increasing in recent years. A recent study performed in UK using real world data showed an increase of incident TT prescriptions from less than 1% in 2001 to 26% in 2016, and an increase in prevalent TT prescription from 1% to 41% in the same period.9 A similar increase was observed in Spain, where a previous epidemiological study by Barrecheguren et al.8 showed that 12% of newly diagnosed COPD patients initiated TT during 2006–2012, which increased to 17% of incident TT during 2011–2015 in our study. Similarly, our study found 18% of patients eligible to be in the study to be receiving TT. Although this percentage is still lower than that observed in other international studies,6,7,21,22 findings support a consistent trend of increasing TT prescription in Primary Care.
Guidelines published during the study period recommended the use of TT in patients with severe disease, frequent exacerbations and persistent symptoms who were not adequately controlled with LABA/LAMA or ICS/LABA combination.2 These recommendations are supported by accumulated evidence from clinical trials showing the benefits of TT compared with dual or monotherapy in more severe patients with exacerbations.13,14 However, real life data indicate that TT prescription patterns often differ from the current recommendations and there has been a reported overuse of TT in different levels of health care, from Primary Care to hospital-based respiratory clinics.4,5,7,20–22 Interestingly, the majority of patients who initiated TT in our study population were classified as GOLD A/B, in whom TT is not recommended. This finding is not unique to Spain, as previous works performed in the UK,21 the US,7 central and Eastern Europe,23 Latin America24 and Japan25 have also shown the over-prescription of TT in patients who are not frequent exacerbators and belong to the GOLD groups A or B. These findings suggest that primary care physicians may rely more on symptoms than exacerbations for their decisions to step up pharmacologic therapy. This hypothesis is supported by several studies that reported progression to TT with no prior exacerbations history,6,7,11 including a recent study by Landis et al.,26 which found that dyspnea was the main driver for therapy changes in COPD patients in Primary Care. However, there is some evidence for the use of TT irrespective of reported exacerbation history. For example, subgroup analyses from the KRONOS trial suggest a benefit of TT over dual therapy even in patients with no exacerbation in the past year.27
The Spanish COPD guidelines3 at the time of the study recommended the use of ICS in patients identified as ACO and/or frequent exacerbators. It is unlikely that the high use of TT was due to a high prevalence of ACO, because only 5.6% of patients were identified as ACO and the prevalence of high blood eosinophil counts was not high enough to justify the use of TT. It is of note, however, that the mean value of blood eosinophil concentrations for our population were relatively high at 247cell/μl. This is similar to a previous study in Spain reporting a mean value of 254cell/μl in a population of 57,000 unselected patients with COPD at all levels of severity.28 Recently, Kolsum et al.29 obtained a mean eosinophil count of 210cell/μl in patients with COPD, which was significantly higher than counts observed in smokers without COPD and health controls.
Our results highlight prescription of ICS-combined regimens and a later escalation to TT, irrespective of exacerbation frequency.6,11,20,26,30–33 These findings may indicate however, that such use may be warranted for some patients and may be reflective of underreporting of exacerbations or control of exacerbations with ICS therapy. However, if used when not appropriate, prior therapy with ICS-containing regimens could result in limited clinical benefits. Consequently, GPs may decide to escalate treatment to TT instead of changing to a non ICS-containing regimen to control disease.
Interestingly, around 20% of patients who initiated TT had no history of previous COPD medication. A similar percentage has been observed in other studies8,34 and indicates again a deviation from treatment recommendations.2,3
We observed that the probability of de-escalation following TT within 5 years is around 50%, but particularly in patients with more severe disease (frequent exacerbators and patients with ACOS) and during the first year. This unexpected result may be due to lack of efficacy despite receiving TT,26 or on the contrary, due to an effective disease stability achieved after TT.35–37 In addition, we cannot rule out that more frequent contacts of severe patients with the healthcare providers may increase the probability of treatment changes, including de-escalation from TT. Unfortunately, we do not have enough clinical information to address the reason for de-escalation; long term follow up and complete sequencing of treatments after TT initiation may be warranted to best investigate reasons for change in treatment patterns.
In a number of cases, step down consisted of discontinuation of a LABD with persistence of the ICS component. This is consistent with other studies38 but contrasts clinical trials demonstrating that ICS can be safely discontinued in some patients with stable COPD, particularly in non-exacerbators with low blood eosinophil counts.39 We observed 6% probability of complete discontinuation of TT within 5 years, especially in less severe patients,40 which is not consistent with current recommendations that suggest LABD treatment in symptomatic COPD.2,3
The current study has some limitations; as in most real word data studies, caution should be taken when interpreting results relating to choice of therapy as the full context of the decision is not recorded. Full categorization of COPD patients was not possible, as symptoms data, including CAT scores and mMRC are not available in SIDIAP and information on lung function is limited; therefore, GOLD categorisation was only possible in two groups A/B or C/D. Although we used a validated algorithm to identify exacerbations, the risk of underreporting still exists and underreporting of exacerbations by patients is also is well documented in the literature. In addition, exacerbations leading to hospitalization or those treated directly by respiratory specialists were not available; however, these episodes are less frequent in and the majority of these episodes can be captured based on prescriptions following the exacerbation episode. Last, exacerbations were only considered over a 12-month period, longer-term monitoring could be useful to further understand exacerbation frequency and treatment patterns. In any case, if underreporting exists, it should affect equally to all subgroups and should not affect significantly the conclusions of the study. Since new evidence on efficacy and safety of inhaled medication is available and guidelines have been modified, it would be relevant to repeat the study to see if there has been any change in the trends observed.
ConclusionsIn conclusion, our results suggest that there is extensive use of TT in primary care, and that the use of TT is not consistent with guideline recommendations. Most patients initiating treatment with TT continue on the same treatment over time regardless of severity of disease. Step down was most frequent in severe patients, while discontinuation was most common among patients with less severe disease. The findings suggest that further research into the drivers of TT prescription in clinical practice would be beneficial.
Authors’ contributionsDL, ND, AB conceived and designed the study. MMo and IS coordinated statistical analyses. MM and MMo wrote manuscript. All authors contributed to study design and analysis, drafting, reviewing, and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Conflict of interestMarc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, pH Pharma, Novartis, Sanofi and Grifols and research grants from GlaxoSmithKline and Grifols. Miriam Barrecheguren has received speaker fees from Grifols, Menarini, CSL Behring, GSK and consulting fees from GSK and Novartis. D. Lambrelli, N. Dhalwani and A. Booth are employees of Evidera. The authors confirm there are no other conflicts of interest to report.
The current study has been funded by an unrestricted grant from AstraZeneca who was also involved in study conception and protocol development. Alexa Nuñez is the recipient of a Rio Hortega contract in the 2019 Strategic Action Health Call from the Instituto de Salud Carlos III for the years 2020–2022.